An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia
- PMID: 11303007
- PMCID: PMC1729738
- DOI: 10.1136/heart.85.5.544
An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia
Abstract
Background: Omega-3 fatty acids, such as those present in fish oil, have been reported to prolong life in myocardial infarction survivors. These fatty acids can decrease serum triglyceride concentrations, but so far the doses used in trials examining their effects on coronary end points have had only minimal triglyceride lowering effects.
Objective: To examine the triglyceride lowering effectiveness, safety, and tolerability of Omacor, a concentrate of omega-3, long chain, polyunsaturated fatty acids from fish oil (84% of the total as opposed to an average of 35% in fish oil) over one year in patients with established coronary heart disease (CHD) and persisting hypertriglyceridaemia, despite receiving simvastatin in doses similar to those employed in the Scandinavian simvastatin survival study.
Subjects and methods: 59 patients with CHD, receiving simvastatin 10-40 mg daily with serum triglycerides > 2.3 mmol/l, were randomised to receive Omacor 2 g twice a day or placebo for 24 weeks in a double blind trial. Forty six patients accepted the offer of active treatment for a further 24 weeks in an open phase of the trial.
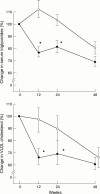
Results: There was a sustained significant decrease in serum triglycerides by 20-30% (p < 0.005) and in very low density lipoprotein (VLDL) cholesterol by 30-40% (p < 0.005) in patients receiving active Omacor at three, six, and 12 months compared either to baseline or placebo. Omacor did not have any deleterious effect on low density or high density lipoprotein cholesterol or on biochemical and haematological safety tests. There was no adverse effect on glycaemic control in patients with diabetes, who showed a decrease in serum triglyceride, which was at least as great as in non-diabetic patients. One patient receiving placebo died of acute myocardial infarction. Three patients withdrew from the trial (two on placebo and one on active treatment). Omacor was generally well tolerated.
Conclusion: Omacor was found to be a safe and effective means of lowering serum triglycerides over one year in patients with CHD and combined hyperlipidaemia, whose triglycerides remained elevated despite simvastatin treatment.
Figures
Similar articles
-
A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Assess the Efficacy and Safety of Ethyl-Ester Omega-3 Fatty Acid in Taiwanese Hypertriglyceridemic Patients.J Atheroscler Thromb. 2017 Mar 1;24(3):275-289. doi: 10.5551/jat.34231. Epub 2016 Sep 6. J Atheroscler Thromb. 2017. PMID: 27600795 Free PMC article. Clinical Trial.
-
Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study.Clin Ther. 2007 Jul;29(7):1354-67. doi: 10.1016/j.clinthera.2007.07.018. Clin Ther. 2007. PMID: 17825687 Clinical Trial.
-
The effect of a 12-week course of omega-3 polyunsaturated fatty acids on lipid parameters in hypertriglyceridemic adult HIV-infected patients undergoing HAART: a randomized, placebo-controlled pilot trial.Clin Ther. 2012 Jan;34(1):67-76. doi: 10.1016/j.clinthera.2011.12.001. Epub 2011 Dec 31. Clin Ther. 2012. PMID: 22212377 Clinical Trial.
-
Omacor and omega-3 fatty acids for treatment of coronary artery disease and the pleiotropic effects.Am J Ther. 2014 Jan-Feb;21(1):56-66. doi: 10.1097/MJT.0b013e31822b5603. Am J Ther. 2014. PMID: 21975796 Review.
-
Omacor (prescription omega-3-acid ethyl esters 90): From severe rhythm disorders to hypertriglyceridemia.Adv Ther. 2009 Jul;26(7):675-90. doi: 10.1007/s12325-009-0045-2. Epub 2009 Jul 27. Adv Ther. 2009. PMID: 19629408 Review.
Cited by
-
The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials.Front Nutr. 2022 Oct 13;9:1039056. doi: 10.3389/fnut.2022.1039056. eCollection 2022. Front Nutr. 2022. PMID: 36313109 Free PMC article.
-
Role of omega-3 ethyl ester concentrate in reducing sudden cardiac death following myocardial infarction and in management of hypertriglyceridemia: an Indian consensus statement.Indian Heart J. 2012 Sep-Oct;64(5):503-7. doi: 10.1016/j.ihj.2012.08.004. Epub 2012 Aug 27. Indian Heart J. 2012. PMID: 23102390 Free PMC article.
-
Dose-dependent effects of docosahexaenoic acid supplementation on blood lipids in statin-treated hyperlipidaemic subjects.Lipids. 2007 Mar;42(2):109-15. doi: 10.1007/s11745-006-3014-4. Epub 2007 Feb 8. Lipids. 2007. PMID: 17393216 Clinical Trial.
-
Association between polymorphisms in the fatty acid desaturase gene cluster and the plasma triacylglycerol response to an n-3 PUFA supplementation.Nutrients. 2012 Aug;4(8):1026-41. doi: 10.3390/nu4081026. Epub 2012 Aug 17. Nutrients. 2012. PMID: 23016130 Free PMC article.
-
A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triglyceride levels.Vasc Health Risk Manag. 2006;2(3):251-62. doi: 10.2147/vhrm.2006.2.3.251. Vasc Health Risk Manag. 2006. PMID: 17326331 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
