Cardiovascular magnetic resonance
- PMID: 11303017
- PMCID: PMC1729746
- DOI: 10.1136/heart.85.5.581
Cardiovascular magnetic resonance
Figures
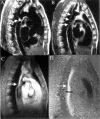
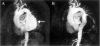
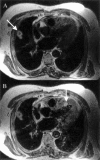

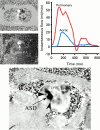

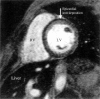
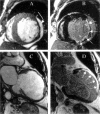
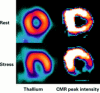
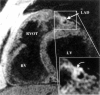
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
