Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients
- PMID: 11303137
- PMCID: PMC1421284
- DOI: 10.1097/00000658-200104000-00010
Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients
Abstract
Objective: To evaluate the prophylactic use of enteral fluconazole to prevent invasive candidal infections in critically ill surgical patients.
Summary background data: Invasive fungal infections are increasingly common in the critically ill, especially in surgical patients. Although fungal prophylaxis has been proven effective in certain high-risk patients such as bone marrow transplant patients, few studies have focused on surgical patients and prevention of fungal infection.
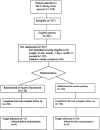
Methods: The authors conducted a prospective, randomized, placebo-controlled trial in a single-center, tertiary care surgical intensive care unit (ICU). A total of 260 critically ill surgical patients with a length of ICU stay of at least 3 days were randomly assigned to receive either enteral fluconazole 400 mg or placebo per day during their stay in the surgical ICU at Johns Hopkins Hospital.
Results: The primary end point was the time to occurrence of fungal infection during the surgical ICU stay, with planned secondary analysis of patients "on-therapy" and alternate definitions of fungal infections. In a time-to-event analysis, the risk of candidal infection in patients receiving fluconazole was significantly less than the risk in patients receiving placebo. After adjusting for potentially confounding effects of the Acute Physiology and Chronic Health Evaluation (APACHE) III score, days to first dose, and fungal colonization at enrollment, the risk of fungal infection was reduced by 55% in the fluconazole group. No difference in death rate was observed between patients receiving fluconazole and those receiving placebo.
Conclusions: Enteral fluconazole safely and effectively decreased the incidence of fungal infections in high-risk, critically ill surgical patients.
Figures
References
-
- Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 1995; 20: 1526–1530. - PubMed
-
- Wey SB, Mori M, Pfaller MA, et al. Risk factors for hospital-acquired candidemia. Arch Intern Med 1989; 149: 2349–2353. - PubMed
-
- Bross J, Talbot GH, Maislin G, et al. Risk factors for nosocomial candidemia: a case-control study. Am J Med 1989; : 614–620. - PubMed
-
- Komshian SV, Uwaydah A, Sobel JD, et al. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev Infect Dis 1989; 11: 379–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical