Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells
- PMID: 11304549
- PMCID: PMC2193402
- DOI: 10.1084/jem.193.8.881
Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells
Abstract
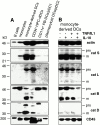
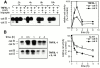
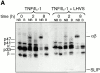
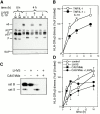
Endo/lysosomal proteases control two key events in antigen (Ag) presentation: the degradation of protein Ag and the generation of peptide-receptive major histocompatibility complex (MHC) class II molecules. Here we show that the proinflammatory cytokines tumor necrosis factor alpha and interleukin (IL)-1beta rapidly increase the activity of cathepsin (cat) S and catB in human dendritic cells (DCs). As a consequence, a wave of MHC class II sodium dodecyl sulfate stable dimer formation ensues in a catS-dependent fashion. In contrast, the antiinflammatory cytokine IL-10 renders DCs incapable of upregulating catS and catB activity and in fact, attenuates the level of both enzymes. Suppressed catS and catB activity delays MHC class II sodium dodecyl sulfate stable dimer formation and impairs Ag degradation. In DCs exposed to tetanus toxoid, IL-10 accordingly reduces the number of MHC class II-peptide complexes accessible to tetanus toxoid-specific T cell receptors, as analyzed by measuring T cell receptor downregulation in Ag-specific T cell clones. Thus, the control of protease activity by pro- and antiinflammatory cytokines is an essential feature of the Ag presentation properties of DCs.
Figures







References
-
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. - PubMed
-
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. - PubMed
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Morel A.S., Quaratino S., Douek D.C., Londei M. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cellsdefinition of a maturative step. Eur. J. Immunol. 1997;27:26–34. - PubMed
-
- Villadangos J.A., Ploegh H.L. Proteolysis in MHC class II antigen presentationwho's in charge? Immunity. 2000;12:233–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

