The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system
- PMID: 11304557
- PMCID: PMC2193406
- DOI: 10.1084/jem.193.8.967
The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system
Abstract
Although it is clear that the function of CD40 on peripheral hematopoietic cells is pivotal to the development of autoimmunity, the function of CD40 in autoimmune disease outside this compartment is unresolved. In a model of experimental autoimmune encephalomyelitis (EAE), evidence is presented that CD40-CD154 interactions within the central nervous system (CNS) are critical determinants of disease development and progression. Using bone marrow (BM) chimeric mice, the data suggest that the lack of expression of CD40 by CNS-resident cells diminishes the intensity and duration of myelin oligodendrocyte glycoprotein (MOG)-induced EAE and also reduces the degree of inflammatory cell infiltrates into the CNS. Although CNS inflammation is compromised in the CD40(+/+)-->CD40(-/-) BM chimeric mice, the restricted CD40 expression had no impact on peripheral T cell priming or recall responses. Analysis of RNA expression levels within the CNS demonstrated that encephalitogenic T cells, which entered a CNS environment in which CD40 was absent from parenchymal microglia, could not elicit the expression of chemokines within the CNS. These data provide evidence that CD40 functions outside of the systemic immune compartment to amplify organ-specific autoimmunity.
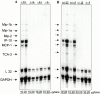
Figures





References
-
- Grewal I.S., Flavell R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. - PubMed
-
- Mackey M.F., Barth R.J.J., Noelle R.J. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J. Leukoc. Biol. 1998;63:418–428. - PubMed
-
- Durie F.H., Fava R.A., Foy T.M., Aruffo A., Ledbetter J.A., Noelle R.J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–1330. - PubMed
-
- Sadlack B., Lohler J., Schorle H., Klebb G., Haber H., Sickel E., Noelle R.J., Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 1995;25:3053–3059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

