Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs
- PMID: 11304559
- PMCID: PMC2193400
- DOI: 10.1084/jem.193.8.981
Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs
Abstract
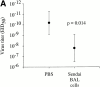
Although CD4(+) T cells have been shown to mediate protective cellular immunity against respiratory virus infections, the underlying mechanisms are poorly understood. For example, although phenotypically distinct populations of memory CD4(+) T cells have been identified in different secondary lymphoid tissues, it is not known which subpopulations mediate protective cellular immunity. In this report, we demonstrate that virus-specific CD4(+) T cells persist in the lung tissues and airways for several months after Sendai virus infection of C57BL/6 mice. A large proportion of these cells possess a highly activated phenotype (CD44(hi), CD62L(lo), CD43(hi), and CD25(hi)) and express immediate effector function as indicated by the production of interferon gamma after a 5-h restimulation in vitro. Furthermore, intratracheal adoptive transfer of lung memory cells into beta2m-deficient mice demonstrated that lung-resident virus-specific CD4(+) T cells mediated a substantial degree of protection against secondary virus infection. Taken together, these data demonstrate that activated memory CD4(+) T cells persisting at mucosal sites play a critical role in mediating protective cellular immunity.
Figures



Comment in
-
Memory CD4 T Cell Distribution and Recall Function in Nonlymphoid Organs.J Immunol. 2023 Jun 1;210(11):1627-1628. doi: 10.4049/jimmunol.2300062. J Immunol. 2023. PMID: 37987803 No abstract available.
References
-
- Doherty P.C., Topham D.J., Tripp R.A., Cardin R.D., Brooks J.W., Stevenson P.G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 1997;159:105–117. - PubMed
-
- Kast W.M., Bronkhorst A.M., de Waal L.P., Melief C.J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J. Exp. Med. 1986;164:723–738. - PMC - PubMed
-
- Zhong W., Marshall D., Coleclough C., Woodland D.L. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J. Immunol. 2000;164:3274–3282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

