Direct competition between Brinker and Drosophila Mad in Dpp target gene transcription
- PMID: 11306550
- PMCID: PMC1083865
- DOI: 10.1093/embo-reports/kve068
Direct competition between Brinker and Drosophila Mad in Dpp target gene transcription
Abstract
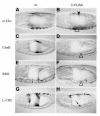
Brinker is a nuclear protein that antagonizes Dpp signalling in Drosophila. Its expression is negatively regulated by Dpp. Here, we show that Brinker represses Ultrabithorax (Ubx) in the embryonic midgut, a HOX gene that activates, and responds to, the localized expression of Dpp during endoderm induction. We find that the functional target for Brinker repression coincides with the Dpp response sequence in the Ubx midgut enhancer, namely a tandem of binding sites for the Dpp effector Mad. We show that Brinker efficiently competes with Mad in vitro, preventing the latter from binding to these sites. Brinker also competes with activated Mad in vivo, blocking the stimulation of the Ubx enhancer in response to simultaneous Dpp signalling. These results indicate how Brinker acts as a dominant repressor of Dpp target genes, and explain why Brinker is a potent antagonist of Dpp.
Figures




Similar articles
-
The transcriptional repressor Brinker antagonizes Wingless signaling.Genes Dev. 2002 Jul 15;16(14):1828-38. doi: 10.1101/gad.230002. Genes Dev. 2002. PMID: 12130542 Free PMC article.
-
Repression of dpp targets by binding of brinker to mad sites.J Biol Chem. 2001 May 25;276(21):18216-22. doi: 10.1074/jbc.M101365200. Epub 2001 Mar 21. J Biol Chem. 2001. PMID: 11262410
-
Transcriptional repression due to high levels of Wingless signalling.EMBO J. 1998 Dec 1;17(23):7021-32. doi: 10.1093/emboj/17.23.7021. EMBO J. 1998. PMID: 9835654 Free PMC article.
-
The zinc finger protein schnurri acts as a Smad partner in mediating the transcriptional response to decapentaplegic.Dev Biol. 2000 Nov 15;227(2):373-87. doi: 10.1006/dbio.2000.9901. Dev Biol. 2000. PMID: 11071761
-
TGF-beta family signal transduction in Drosophila development: from Mad to Smads.Dev Biol. 1999 Jun 15;210(2):251-68. doi: 10.1006/dbio.1999.9282. Dev Biol. 1999. PMID: 10357889 Review.
Cited by
-
The complex spatio-temporal regulation of the Drosophila myoblast attractant gene duf/kirre.PLoS One. 2009 Sep 9;4(9):e6960. doi: 10.1371/journal.pone.0006960. PLoS One. 2009. PMID: 19742310 Free PMC article.
-
Bone morphogenetic protein signaling: the pathway and its regulation.Genetics. 2024 Feb 7;226(2):iyad200. doi: 10.1093/genetics/iyad200. Genetics. 2024. PMID: 38124338 Free PMC article. Review.
-
Wingless directly represses DPP morphogen expression via an armadillo/TCF/Brinker complex.PLoS One. 2007 Jan 3;2(1):e142. doi: 10.1371/journal.pone.0000142. PLoS One. 2007. PMID: 17206277 Free PMC article.
-
Manipulating the sensitivity of signal-induced repression: quantification and consequences of altered brinker gradients.PLoS One. 2013 Aug 8;8(8):e71224. doi: 10.1371/journal.pone.0071224. eCollection 2013. PLoS One. 2013. PMID: 23951114 Free PMC article.
-
The transcriptional repressor Brinker antagonizes Wingless signaling.Genes Dev. 2002 Jul 15;16(14):1828-38. doi: 10.1101/gad.230002. Genes Dev. 2002. PMID: 12130542 Free PMC article.
References
-
- Bienz M. (1996) Induction of the endoderm in Drosophila. Annu. Rev. Cell Dev. Biol., 7, 113–119.
-
- Bienz M. and Tremml, G. (1988) Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion. Nature, 333, 576–578. - PubMed
-
- Brand A.H. and Perrimon, N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. - PubMed
-
- Brook W.J. and Cohen, S.M. (1996) Antagonistic interactions between wg and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila Leg. Science, 273, 1373–1377. - PubMed
-
- Campbell G. and Tomlinson, A. (1999) Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell, 96, 553–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

