Expression of IL-5 alters bone metabolism and induces ossification of the spleen in transgenic mice
- PMID: 11306598
- PMCID: PMC199553
- DOI: 10.1172/JCI11232
Expression of IL-5 alters bone metabolism and induces ossification of the spleen in transgenic mice
Abstract
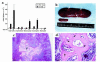
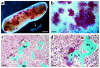
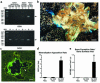
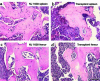
We have developed a transgenic mouse line, NJ.1638, which expresses high levels of IL-5 from T cells, with profound hematological consequences. Eosinophils comprise more than 60% of circulating white blood cells in these animals, with the total peripheral white blood cell counts increasing more than 40-fold relative to wild-type littermates. This extraordinary proliferative capacity is sustained by expanded sites of extramedullary hematopoiesis and is accompanied by multifocal, ectopic bone formation in the spleen. Histology of the splenic nodules revealed the presence of osteoid matrices and osteocytes trapped within mineralized trabecular plates. In addition, polarized light microscopy of calcified tissue sections revealed both woven bone and areas of organized lamellar bone. Morphometric assessments demonstrated that both the growth and mineralization of splenic bone occurred at rates nearly an order of magnitude higher than in skeletal bone. Skeletal bone metabolic parameters were also perturbed. We also observed heterotopic ossification of the spleen and perturbation of skeletal bone homeostasis following adoptive engraftment of transgenic marrow to wild-type recipients. These data suggest that IL-5 overexpression mediates bone formation through the mobilization of marrow-derived osteogenic progenitors and/or the inhibition of recruited osteoclasts.
Figures

Similar articles
-
Constitutive overexpression of IL-5 induces extramedullary hematopoiesis in the spleen.Blood. 2003 Feb 1;101(3):863-8. doi: 10.1182/blood-2002-03-0735. Epub 2002 Sep 12. Blood. 2003. PMID: 12393708
-
Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system.Arthritis Rheum. 2006 Nov;54(11):3551-63. doi: 10.1002/art.22175. Arthritis Rheum. 2006. PMID: 17075861
-
Impaired vascular invasion of Cbfa1-deficient cartilage engrafted in the spleen.J Bone Miner Res. 2002 Jul;17(7):1297-305. doi: 10.1359/jbmr.2002.17.7.1297. J Bone Miner Res. 2002. PMID: 12096844
-
The effect of the spleen on compartmental levels and distribution of donor progenitor cells after syngeneic and allogeneic bone marrow transplants.Stem Cells Dev. 2004 Feb;13(1):51-62. doi: 10.1089/154732804773099254. Stem Cells Dev. 2004. PMID: 15068693
-
[The regulation of bone marrow hematopoiesis in splenectomy and splenic autotransplantation in mice].Morfologiia. 1993 May-Jun;104(5-6):73-7. Morfologiia. 1993. PMID: 8012541 Russian.
Cited by
-
Cytokine-mediated immunomodulation of osteoclastogenesis.Bone. 2022 Nov;164:116540. doi: 10.1016/j.bone.2022.116540. Epub 2022 Aug 27. Bone. 2022. PMID: 36031187 Free PMC article. Review.
-
Emerging Roles of Eosinophils in Bone.Curr Osteoporos Rep. 2025 Apr 4;23(1):17. doi: 10.1007/s11914-025-00913-6. Curr Osteoporos Rep. 2025. PMID: 40183859 Free PMC article. Review.
-
GATA1 controls numbers of hematopoietic progenitors and their response to autoimmune neuroinflammation.Blood Adv. 2022 Dec 13;6(23):5980-5994. doi: 10.1182/bloodadvances.2022008234. Blood Adv. 2022. PMID: 36206195 Free PMC article.
-
Splenocytes seed bone marrow of myeloablated mice: implication for atherosclerosis.PLoS One. 2015 Jun 3;10(6):e0125961. doi: 10.1371/journal.pone.0125961. eCollection 2015. PLoS One. 2015. PMID: 26038819 Free PMC article.
-
The FIP1L1-PDGFRA fusion gene cooperates with IL-5 to induce murine hypereosinophilic syndrome (HES)/chronic eosinophilic leukemia (CEL)-like disease.Blood. 2006 May 15;107(10):4071-9. doi: 10.1182/blood-2005-08-3153. Epub 2006 Jan 17. Blood. 2006. PMID: 16418325 Free PMC article.
References
-
- Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. - PubMed
-
- Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999;72:396–410. - PubMed
-
- Andrades JA, et al. A recombinant human TGF-beta1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp Cell Res. 1999;250:485–498. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

