GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter
- PMID: 11306616
- PMCID: PMC6762542
- DOI: 10.1523/JNEUROSCI.21-08-02630.2001
GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter
Abstract
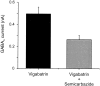
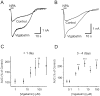
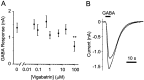
The GABA transporter can reverse with depolarization, causing nonvesicular GABA release. However, this is thought to occur only under pathological conditions. Patch-clamp recordings were made from rat hippocampal neurons in primary cell cultures. Inhibition of GABA transaminase with the anticonvulsant gamma-vinyl GABA (vigabatrin; 0.05-100 microm) resulted in a large leak current that was blocked by bicuculline (50 microm). This leak current occurred in the absence of extracellular calcium and was blocked by the GABA transporter antagonist SKF-89976a (5 microm). These results indicate that vigabatrin induces spontaneous GABA efflux from neighboring cells via reversal of GABA transporters, subsequently leading to the stimulation of GABA(A) receptors on the recorded neuron. The leak current increased slowly over 4 d of treatment with 100 microm vigabatrin, at which time it reached an equivalent conductance of 9.0 +/- 4.9 nS. Blockade of glutamic acid decarboxylase with semicarbazide (2 mm) decreased the leak current that was induced by vigabatrin by 47%. In untreated cells, carrier-mediated GABA efflux did not occur spontaneously but was induced by an increase in [K(+)](o) from 3 to as little as 6 mm. Vigabatrin enhanced this depolarization-evoked nonvesicular GABA release and also enhanced the heteroexchange release of GABA induced by nipecotate. Thus, the GABA transporter normally operates near its equilibrium and can be easily induced to reverse by an increase in cytosolic [GABA] or mild depolarization. We propose that this transporter-mediated nonvesicular GABA release plays an important role in neuronal inhibition under both physiological and pathophysiological conditions and is the target of some anticonvulsants.
Figures







Similar articles
-
Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release.J Neurophysiol. 2003 Apr;89(4):2021-34. doi: 10.1152/jn.00856.2002. Epub 2002 Dec 27. J Neurophysiol. 2003. PMID: 12612025
-
Carrier-mediated GABA release activates GABA receptors on hippocampal neurons.J Neurophysiol. 1998 Jul;80(1):270-81. doi: 10.1152/jn.1998.80.1.270. J Neurophysiol. 1998. PMID: 9658049
-
Slow desensitization regulates the availability of synaptic GABA(A) receptors.J Neurosci. 2000 Nov 1;20(21):7914-21. doi: 10.1523/JNEUROSCI.20-21-07914.2000. J Neurosci. 2000. PMID: 11050111 Free PMC article.
-
GABA transporters and GABA-transaminase as drug targets.Curr Drug Targets CNS Neurol Disord. 2003 Aug;2(4):269-77. doi: 10.2174/1568007033482788. Curr Drug Targets CNS Neurol Disord. 2003. PMID: 12871037 Review.
-
[The neurotransmitter inhibitor GABA, the basis of the mechanism of action of several drugs affecting the central nervous system].An R Acad Nac Med (Madr). 1984;101(4):431-52. An R Acad Nac Med (Madr). 1984. PMID: 6152518 Review. Spanish. No abstract available.
Cited by
-
Local and Interregional Neurochemical Associations Measured by Magnetic Resonance Spectroscopy for Studying Brain Functions and Psychiatric Disorders.Front Psychiatry. 2020 Aug 11;11:802. doi: 10.3389/fpsyt.2020.00802. eCollection 2020. Front Psychiatry. 2020. PMID: 32848957 Free PMC article. Review.
-
Role of Cl- -HCO3- exchanger AE3 in intracellular pH homeostasis in cultured murine hippocampal neurons, and in crosstalk to adjacent astrocytes.J Physiol. 2017 Jan 1;595(1):93-124. doi: 10.1113/JP272470. Epub 2016 Nov 6. J Physiol. 2017. PMID: 27353306 Free PMC article.
-
Influence of glutamate and GABA transport on brain excitatory/inhibitory balance.Exp Biol Med (Maywood). 2021 May;246(9):1069-1083. doi: 10.1177/1535370221989263. Epub 2021 Feb 7. Exp Biol Med (Maywood). 2021. PMID: 33554649 Free PMC article. Review.
-
The effects of volatile anesthetics on the extracellular accumulation of [(3)H]GABA in rat brain cortical slices.Cell Mol Neurobiol. 2014 Jan;34(1):71-81. doi: 10.1007/s10571-013-9988-6. Epub 2013 Oct 1. Cell Mol Neurobiol. 2014. PMID: 24081560 Free PMC article.
-
Expression and localization of Na-driven Cl-HCO(3)(-) exchanger (SLC4A8) in rodent CNS.Neuroscience. 2008 Apr 22;153(1):162-74. doi: 10.1016/j.neuroscience.2008.02.018. Epub 2008 Feb 21. Neuroscience. 2008. PMID: 18359573 Free PMC article.
References
-
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
-
- Belhage B, Hansen GH, Schousboe A. Depolarization by K+ and glutamate activates different neurotransmitter release mechanisms in GABAergic neurons: vesicular versus nonvesicular release of GABA. Neuroscience. 1993;54:1019–1034. - PubMed
-
- Ben-Menachem E, Hamberger A, Mumford J. Effect of long-term vigabatrin therapy on GABA and other amino acid concentrations in the central nervous system—a case study. Epilepsy Res. 1993;16:241–243. - PubMed
-
- Bernath S, Zigmond MJ. Characterization of [3H]GABA release from striatal slices: evidence for a calcium-independent process via the GABA uptake system. Neuroscience. 1988;27:563–570. - PubMed
-
- Bernstein EM, Quick MW. Regulation of γ-aminobutyric acid (GABA) transporters by extracellular GABA. J Biol Chem. 1999;274:889–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
