Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever
- PMID: 11306620
- PMCID: PMC6762538
- DOI: 10.1523/JNEUROSCI.21-08-02669.2001
Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever
Abstract
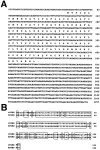
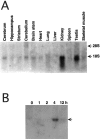
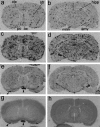
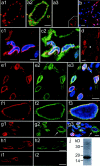
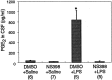
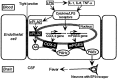
Fever is triggered by an elevation of prostaglandin E(2) (PGE(2)) in the brain. However, the mechanism of its elevation remains unanswered. We herein cloned the rat glutathione-dependent microsomal prostaglandin E synthase (mPGES), the terminal enzyme for PGE(2) biosynthesis, and examined its induction in the rat brain after intraperitoneal injection of pyrogen lipopolysaccharide (LPS). In Northern blot analysis, mPGES mRNA was weakly expressed in the brain under the normal conditions but was markedly induced between 2 and 4 hr after the LPS injection. In situ hybridization study revealed that LPS-induced mPGES mRNA signals were mainly associated with brain blood vessels, especially vein or venular-type ones, in the whole brain area. Immunohistochemical study demonstrated that mPGES-like immunoreactivity was expressed in the perinuclear region of brain endothelial cells, which were identified as von Willebrand factor-positive cells. Furthermore, in the perinuclear region of the endothelial cells, mPGES was colocalized with cyclooxygenase-2 (COX-2), which is the enzyme essential for the production of the mPGES substrate PGH(2). Inhibition of cyclooxygenase-2 activity resulted in suppression of both PGE(2) level in the CSF and fever (Cao et al., 1997), suggesting that the two enzymes were functionally linked and that this link is essential for fever. These results demonstrate that brain endothelial cells play an essential role in the PGE(2) production during fever by expressing COX-2 and mPGES.
Figures

References
-
- Blalock JE. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989;69:1–32. - PubMed
-
- Blatteis CM, Sehic E. Fever: how may circulating pyrogens signal the brain? News Physiol Sci. 1997;12:1–9.
-
- Breder CD, Saper CB. Expression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharide. Brain Res. 1996;713:64–69. - PubMed
-
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Induction by lipopolysaccharide of cyclooxygenase-2 mRNA in rat brain; its possible role in the febrile response. Brain Res. 1995;697:187–196. - PubMed
-
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1β: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
