Slow recovery from inactivation regulates the availability of voltage-dependent Na(+) channels in hippocampal granule cells, hilar neurons and basket cells
- PMID: 11306658
- PMCID: PMC2278538
- DOI: 10.1111/j.1469-7793.2001.0385f.x
Slow recovery from inactivation regulates the availability of voltage-dependent Na(+) channels in hippocampal granule cells, hilar neurons and basket cells
Erratum in
- J Physiol 2001 Jun 15;533 Pt 3:921
Abstract
1. Fundamental to the understanding of CNS function is the question of how individual neurons integrate multiple synaptic inputs into an output consisting of a sequence of action potentials carrying information coded as spike frequency. The availability for activation of neuronal Na(+) channels is critical for this process and is regulated both by fast and slow inactivation processes. Here, we have investigated slow inactivation processes in detail in hippocampal neurons. 2. Slow inactivation was induced by prolonged (10-300 s) step depolarisations to -10 mV at room temperature. In isolated hippocampal dentate granule cells (DGCs), recovery from this inactivation was biexponential, with time constants for the two phases of slow inactivation tau(slow,1) and tau(slow,2) ranging from 1 to 10 s and 20 to 50 s, respectively. Both (slow,1) and tau(slow,2) were related to the duration of prior depolarisation by a power law function of the form tau(t) = a (t/a)b, where t is the duration of the depolarisation, a is a constant kinetic setpoint and b is a scaling power. This analysis yielded values of a = 0.034 s and b = 0.62 for tau(slow,1) and a = 24 s and b = 0.30 for tau(slow,2) in the rat. 3. When a train of action potential-like depolarisations of different frequencies (50, 100, 200 Hz) was used to induce inactivation, a similar relationship was found between the frequency of depolarisation and both tau(slow,1) and tau(slow,2) (a = 0.58 s, b = 0.39 for tau(slow,1) and a = 3.77 s and b = 0.42 for tau(slow,2)). 4. Using nucleated patches from rat hippocampal slices, we have addressed possible cell specific differences in slow inactivation. In fast-spiking basket cells a similar scaling relationship can be found (a = 3.54 s and b = 0.39) as in nucleated patches from DGCs (a = 2.3 s and b = 0.48) and non-fast-spiking hilar neurons (a = 2.57 s and b = 0.49). 5. Likewise, comparison of human and rat granule cells showed that properties of ultra-slow recovery from inactivation are conserved across species. In both species ultra-slow recovery was biexponential with both tau(slow,1) and tau(slow,2) being related to the duration of depolarisation t, with a = 0.63 s and b = 0.44 for tau(slow,1) and a = 25 s and b = 0.37 for tau(slow,2) for the human subject. 6. In summary, we describe in detail how the biophysical properties of Na(+) channels result in a complex interrelationship between availability of sodium channels and membrane potential or action potential frequency that may contribute to temporal integration on a time scale of seconds to minutes in different types of hippocampal neurons.
Figures



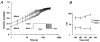
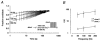


References
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. - PubMed
-
- Black JA, Yokoyama S, Higashida H, Ransom BR, Waxman SG. Sodium channel mRNAs I, II and III in the CNS: cell-specific expression. Molecular Brain Research. 1994;22:275–289. - PubMed
-
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiological Reviews. 1992;72(suppl.):15–48S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

