Respiratory activity in glossopharyngeal, vagus and accessory nerves and pharyngeal constrictors in newborn rat in vitro
- PMID: 11306670
- PMCID: PMC2278554
- DOI: 10.1111/j.1469-7793.2001.0535f.x
Respiratory activity in glossopharyngeal, vagus and accessory nerves and pharyngeal constrictors in newborn rat in vitro
Abstract
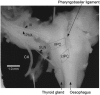
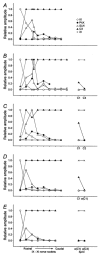
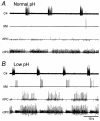
1. Previously, in a brainstem-spinal cord-rib preparation from neonatal rats we demonstrated that a decrement in extracellular pH (from about 7.4 to 7.1) caused expiratory activity in an internal intercostal muscle (IIM) during the first half of the expiratory phase (Ea). As the initial step in finding nerves or muscles firing during the second half of the expiratory phase (Eb), the patterns of activity in the glossopharyngeal, vagus and accessory nerves were examined in the present study. 2. Since the emerging motor rootlets of these three nerves (> 20; collected into about 10 bundles before the jugular foramen) are distributed in a continuous fashion from rostral to caudal levels of the brainstem, visual identification was impossible. Therefore, antidromic compound action potentials evoked by stimulation of the glossopharyngeal nerve (IX), the pharyngeal branch of the vagus nerve (PhX), the superior laryngeal nerve (SLN), the cervical vagus nerve (CX) and the accessory nerve (XI) were recorded from the peripheral stumps of the various rootlets. Nerve rootlets could be categorised into rostral, intermediate and caudal groups (rostIX-XI, intIX-XI, caudIX-XI). The rostIX-XI rootlets showed their largest potential on IX stimulation, while the intIX-XI and caudIX-XI rootlets showed their largest potentials on CX stimulation. The intIX-XI rootlets showed larger potentials on PhX and SLN stimulation than the caudIX-XI rootlets. 3. Activity was recorded simultaneously from the central stumps of the rootlets in the above three groups. Most rootlets showed inspiratory bursts. Under low pH conditions, all representatives of group rostIX-XI, most of intIX-XI and about half of caudIX-XI showed additional bursts during the Ea phase. Groups intIX-XI and caudIX-XI but not rostIX-XI also showed discrete bursts during the Eb phase in some preparations. In general, expiratory activity was prominent in intIX-XI. The spinal branch of XI showed no consistent respiratory activity. 4. Since the intIX-XI rootlets showed Eb bursts and large antidromic potentials on stimulation of PhX and SLN (which innervate the inferior pharyngeal constrictor muscle (IPC)), electromyograms were recorded from the rostral and caudal parts of IPC (rIPC and cIPC). Under low pH conditions, cIPC showed bursts during the Ea and Eb phases, while rIPC showed bursts predominantly during the Eb phase. 5. These results indicate that recording from rIPC would be a useful way of examining the neuronal mechanisms responsible for Eb phase activity.
Figures





Similar articles
-
Compared effects of serotonin on the inspiratory activity of glossopharyngeal, vagal, hypoglossal and cervical motoneurons in neonatal rat brain stem-spinal cord preparations.Neurosci Lett. 1993 Sep 17;160(1):61-4. doi: 10.1016/0304-3940(93)9003-3. Neurosci Lett. 1993. PMID: 8247335
-
Respiratory-related activity of the pharyngeal nerves in the rat.Respir Physiol. 1994 Nov-Dec;98(3):295-304. doi: 10.1016/0034-5687(94)90078-7. Respir Physiol. 1994. PMID: 7899730
-
Pharyngeal motoneurones: respiratory-related activity and responses to laryngeal afferents in the decerebrate cat.Exp Brain Res. 1989;78(2):336-44. doi: 10.1007/BF00228905. Exp Brain Res. 1989. PMID: 2599043
-
The Location of the Parasympathetic Fibres within the Vagus Nerve Rootlets: A Case Report and a Review of the Literature.Stereotact Funct Neurosurg. 2023;101(1):68-71. doi: 10.1159/000528094. Epub 2022 Dec 29. Stereotact Funct Neurosurg. 2023. PMID: 36580909 Free PMC article. Review.
-
[Functional anatomy of the glossopharyngeal, vagus, accessory and hypoglossal cranial nerves].Neurochirurgie. 2009 Apr;55(2):132-5. doi: 10.1016/j.neuchi.2009.01.018. Epub 2009 Mar 21. Neurochirurgie. 2009. PMID: 19304301 Review. French.
Cited by
-
Neuromuscular Specializations of the Human Hypopharyngeal Muscles.Dysphagia. 2021 Oct;36(5):769-785. doi: 10.1007/s00455-020-10212-0. Epub 2020 Nov 7. Dysphagia. 2021. Retraction in: Dysphagia. 2023 Apr;38(2):729. doi: 10.1007/s00455-022-10534-1. PMID: 33159539 Retracted. Review.
-
GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat.J Physiol. 2003 Sep 1;551(Pt 2):617-33. doi: 10.1113/jphysiol.2003.042689. Epub 2003 Aug 8. J Physiol. 2003. PMID: 12909685 Free PMC article.
References
-
- Arata A, Onimaru H, Homma I. Possible synaptic connections of expiratory neurons in the medulla of newborn rat in vitro. NeuroReport. 1998;9:743–746. - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. - PubMed
-
- Barillot JC, Grélot L, Reddad S, Bianchi AL. Discharge patterns of laryngeal motoneurones in the cat: an intracellular study. Brain Research. 1990;509:99–106. - PubMed
-
- Bartlett D, Jr, Remmers JE, Gautier H. Laryngeal regulation of respiratory airflow. Respiration Physiology. 1973;18:194–204. - PubMed
-
- Bianchi AL, Barillot JC. Activity of medullary respiratory neurones during reflexes from the lungs in cats. Respiration Physiology. 1975;25:335–352. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

