Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines
- PMID: 11309113
- PMCID: PMC3922410
- DOI: 10.1046/j.1365-2958.2001.02302.x
Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines
Abstract
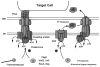
Bacterial conjugation systems are highly promiscuous macromolecular transfer systems that impact human health significantly. In clinical settings, conjugation is exceptionally problematic, leading to the rapid dissemination of antibiotic resistance genes and other virulence traits among bacterial populations. Recent work has shown that several pathogens of plants and mammals - Agrobacterium tumefaciens, Bordetella pertussis, Helicobacter pylori and Legionella pneumophila - have evolved secretion pathways ancestrally related to conjugation systems for the purpose of delivering effector molecules to eukaryotic target cells. Each of these systems exports distinct DNA or protein substrates to effect a myriad of changes in host cell physiology during infection. Collectively, secretion pathways ancestrally related to bacterial conjugation systems are now referred to as the type IV secretion family. The list of putative type IV family members is increasing rapidly, suggesting that macromolecular transfer by these systems is a widespread phenomenon in nature.
Figures


References
-
- Bittinger MA, Gross JA, Widom J, Clardy J, Handelsman J. Rhizobium etli CE3 carries vir gene homologs on a self-transmissible plasmid. Mol Plant–Microbe Interact. 2000;13:1019–1021. - PubMed
-
- Burns DL. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. - PubMed
-
- Cabezon E, Sastre JI, de la Cruz F. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

