OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes
- PMID: 11309411
- PMCID: PMC2169454
- DOI: 10.1083/jcb.153.2.295
OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes
Abstract
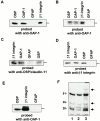
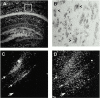
Oligodendrocyte-specific protein (OSP)/claudin-11 is a major component of central nervous system myelin and forms tight junctions (TJs) within myelin sheaths. TJs are essential for forming a paracellular barrier and have been implicated in the regulation of growth and differentiation via signal transduction pathways. We have identified an OSP/claudin-11-associated protein (OAP)1, using a yeast two-hybrid screen. OAP-1 is a novel member of the tetraspanin superfamily, and it is widely expressed in several cell types, including oligodendrocytes. OAP-1, OSP/claudin-11, and beta1 integrin form a complex as indicated by coimmunoprecipitation and confocal immunocytochemistry. Overexpression of OSP/claudin-11 or OAP-1 induced proliferation in an oligodendrocyte cell line. Anti-OAP-1, anti-OSP/claudin-11, and anti-beta1 integrin antibodies inhibited migration of primary oligodendrocytes, and migration was impaired in OSP/claudin-11-deficient primary oligodendrocytes. These data suggest a role for OSP/claudin-11, OAP-1, and beta1 integrin complex in regulating proliferation and migration of oligodendrocytes, a process essential for normal myelination and repair.
Figures







References
-
- Blaschuk K.L., Frost E.E., Ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127:1961–1969. - PubMed
-
- Bronner-Fraser M. Segregation of cell lineage in the neural crest. Curr. Opin. Genet. Dev. 1993;3:641–647. - PubMed
-
- Bronstein J.M., Popper P., Micevych P.E., Farber D.B. Isolation and characterization of a novel oligodendrocyte-specific protein. Neurology. 1996;47:772–778. - PubMed
-
- Bronstein J.M., Micevych P.E., Chen K. Oligodendrocyte-specific protein (OSP) is a major component of CNS myelin. J. Neurosci. Res. 1997;50:713–720. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

