Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin
- PMID: 11309416
- PMCID: PMC2169459
- DOI: 10.1083/jcb.153.2.351
Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin
Abstract
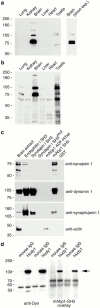
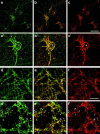
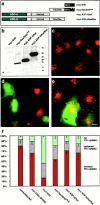
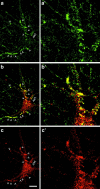
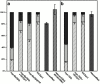
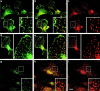
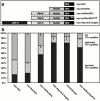
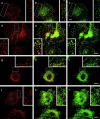
The actin cytoskeleton has been implicated in endocytosis, yet few molecular links to the endocytic machinery have been established. Here we show that the mammalian F-actin-binding protein Abp1 (SH3P7/HIP-55) can functionally link the actin cytoskeleton to dynamin, a GTPase that functions in endocytosis. Abp1 binds directly to dynamin in vitro through its SH3 domain. Coimmunoprecipitation and colocalization studies demonstrated the in vivo relevance of this interaction. In neurons, mammalian Abp1 and dynamin colocalized at actin-rich sites proximal to the cell body during synaptogenesis. In fibroblasts, mAbp1 appeared at dynamin-rich sites of endocytosis upon growth factor stimulation. To test whether Abp1 functions in endocytosis, we overexpressed several Abp1 constructs in Cos-7 cells and assayed receptor-mediated endocytosis. While overexpression of Abp1's actin-binding modules did not interfere with endocytosis, overexpression of the SH3 domain led to a potent block of transferrin uptake. This implicates the Abp1/dynamin interaction in endocytic function. The endocytosis block was rescued by cooverexpression of dynamin. Since the addition of the actin-binding modules of Abp1 to the SH3 domain construct also fully restored endocytosis, Abp1 may support endocytosis by combining its SH3 domain interactions with cytoskeletal functions in response to signaling cascades converging on this linker protein.
Figures

References
-
- Ahn S., Maudsley S., Luttrell L.M., Lefkowitz R.J., Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 1999;274:1185–1188. - PubMed
-
- Brodin L., Löw P., Shupliakov O. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr. Opin. Neurobiol. 2000;10:312–320. - PubMed
-
- Clark S.G., Stern M.J., Horvitz H.R. C. elegans cell-signaling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

