Structure-based design of selective and potent G quadruplex-mediated telomerase inhibitors
- PMID: 11309493
- PMCID: PMC33125
- DOI: 10.1073/pnas.081560598
Structure-based design of selective and potent G quadruplex-mediated telomerase inhibitors
Abstract
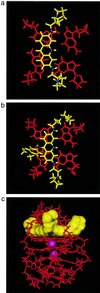
The telomerase enzyme is a potential therapeutic target in many human cancers. A series of potent inhibitors has been designed by computer modeling, which exploit the unique structural features of quadruplex DNA. These 3,6,9-trisubstituted acridine inhibitors are predicted to interact selectively with the human DNA quadruplex structure, as a means of specifically inhibiting the action of human telomerase in extending the length of single-stranded telomeric DNA. The anilino substituent at the 9-position of the acridine chromophore is predicted to lie in a third groove of the quadruplex. Calculated relative binding energies predict enhanced selectivity compared with earlier 3,6-disubstituted compounds, as a result of this substituent. The ranking order of energies is in accord with equilibrium binding constants for quadruplex measured by surface plasmon resonance techniques, which also show reduced duplex binding compared with the disubstituted compounds. The 3,6,9-trisubstututed acridines have potent in vitro inhibitory activity against human telomerase, with EC(50) values of up to 60 nM.
Figures


Similar articles
-
Trisubstituted acridine derivatives as potent and selective telomerase inhibitors.J Med Chem. 2003 Oct 9;46(21):4463-76. doi: 10.1021/jm0308693. J Med Chem. 2003. PMID: 14521409
-
Human telomerase inhibition by regioisomeric disubstituted amidoanthracene-9,10-diones.J Med Chem. 1998 Nov 19;41(24):4873-84. doi: 10.1021/jm981067o. J Med Chem. 1998. PMID: 9822556
-
Trisubstituted acridines as G-quadruplex telomere targeting agents. Effects of extensions of the 3,6- and 9-side chains on quadruplex binding, telomerase activity, and cell proliferation.J Med Chem. 2006 Jan 26;49(2):582-99. doi: 10.1021/jm050555a. J Med Chem. 2006. PMID: 16420044
-
Quadruplex DNA crystal structures and drug design.Biochimie. 2008 Aug;90(8):1184-96. doi: 10.1016/j.biochi.2008.03.003. Epub 2008 Mar 19. Biochimie. 2008. PMID: 18395014 Review.
-
Fluorescence-based melting assays for studying quadruplex ligands.Methods. 2007 Jun;42(2):183-95. doi: 10.1016/j.ymeth.2006.10.004. Methods. 2007. PMID: 17472900 Review.
Cited by
-
Quadruplex ligands may act as molecular chaperones for tetramolecular quadruplex formation.Nucleic Acids Res. 2007;35(8):2483-93. doi: 10.1093/nar/gkm098. Epub 2007 Mar 29. Nucleic Acids Res. 2007. PMID: 17395639 Free PMC article.
-
Drug discovery of small molecules targeting the higher-order hTERT promoter G-quadruplex.PLoS One. 2022 Jun 16;17(6):e0270165. doi: 10.1371/journal.pone.0270165. eCollection 2022. PLoS One. 2022. PMID: 35709230 Free PMC article.
-
G-quadruplexes as potential therapeutic targets for embryonal tumors.Molecules. 2013 Oct 10;18(10):12500-37. doi: 10.3390/molecules181012500. Molecules. 2013. PMID: 24152672 Free PMC article. Review.
-
Radiolytic reduction characteristics of artificial oligodeoxynucleotides possessing 2-oxoalkyl group or disulfide bonds.J Nucleic Acids. 2011;2011:816207. doi: 10.4061/2011/816207. Epub 2011 Aug 8. J Nucleic Acids. 2011. PMID: 21860782 Free PMC article.
-
V-shaped dinuclear Pt(II) complexes: selective interaction with human telomeric G-quadruplex and significant inhibition towards telomerase.Sci Rep. 2013;3:2060. doi: 10.1038/srep02060. Sci Rep. 2013. PMID: 23792883 Free PMC article.
References
-
- McEachern M J, Krauskopf A, Blackburn E H. Annu Rev Genet. 2000;34:331–358. - PubMed
-
- Williamson J R. Annu Rev Biophys Biomol Struct. 1994;23:703–730. - PubMed
-
- Griffith J D, Comeau L, Rosenfield S, Stansel R M, Bianchi A, Moss H, de Lange T. Cell. 1999;97:503–514. - PubMed
-
- Bryan T M, Cech T R. Curr Opin Cell Biol. 1999;11:318–324. - PubMed
-
- Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

