Characterization of a high-voltage-activated IA current with a role in spike timing and locomotor pattern generation
- PMID: 11309504
- PMCID: PMC33200
- DOI: 10.1073/pnas.091096198
Characterization of a high-voltage-activated IA current with a role in spike timing and locomotor pattern generation
Abstract
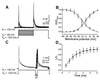
Transient A-type K+ channels (I(A)) in neurons have been implicated in the delay of the spike onset and the decrease in the firing frequency. Here we have characterized biophysically and pharmacologically an I(A) current in lamprey locomotor network neurons that is activated by suprathreshold depolarization and is specifically blocked by catechol at 100 microM. The biophysical properties of this current are similar to the mammalian Kv3.4 channel. The role of the I(A) current both in single neuron firing and in locomotor pattern generation was analyzed. The I(A) current facilitates Na+ channel recovery from inactivation and thus sustains repetitive firing. The role of the I(A) current in motor pattern generation was examined by applying catechol during fictive locomotion induced by N-methyl-d-aspartate. Blockade of this current increased the locomotor burst frequency and decreased the firing of motoneurons. Although an alternating motor pattern could still be generated, the cycle duration was less regular, with ventral roots bursts failing on some cycles. Our results thus provide insights into the contribution of a high-voltage-activated I(A) current to the regulation of firing properties and motor coordination in the lamprey spinal cord.
Figures

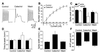


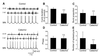
References
-
- Arshavsky Y I, Orlovsky G N, Panchin Y V, Roberts A, Soffe S R. Trends Neurosci. 1993;16:227–232. - PubMed
-
- Marder E, Calabrese R L. Physiol Rev. 1996;76:687–717. - PubMed
-
- Grillner S, Ekeberg Ö, El Manira A, Lansner A, Parker D, Tegnér J, Wallén P. Brain Res Rev. 1998;26:184–197. - PubMed
-
- Delgado-Lezama R, Hounsgaard J. Prog Brain Res. 1999;123:57–63. - PubMed
-
- El Manira A, Tegnér J, Grillner S. J Neurophysiol. 1994;72:1852–1861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

