Mitochondrial abnormalities in Alzheimer's disease
- PMID: 11312286
- PMCID: PMC6762571
- DOI: 10.1523/JNEUROSCI.21-09-03017.2001
Mitochondrial abnormalities in Alzheimer's disease
Abstract
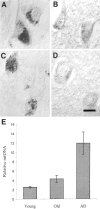
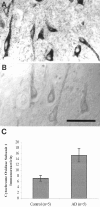
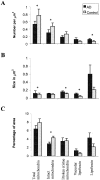
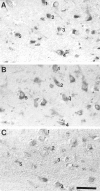
The finding that oxidative damage, including that to nucleic acids, in Alzheimer's disease is primarily limited to the cytoplasm of susceptible neuronal populations suggests that mitochondrial abnormalities might be part of the spectrum of chronic oxidative stress of Alzheimer's disease. In this study, we used in situ hybridization to mitochondrial DNA (mtDNA), immunocytochemistry of cytochrome oxidase, and morphometry of electron micrographs of biopsy specimens to determine whether there are mitochondrial abnormalities in Alzheimer's disease and their relationship to oxidative damage marked by 8-hydroxyguanosine and nitrotyrosine. We found that the same neurons showing increased oxidative damage in Alzheimer's disease have a striking and significant increase in mtDNA and cytochrome oxidase. Surprisingly, much of the mtDNA and cytochrome oxidase is found in the neuronal cytoplasm and in the case of mtDNA, the vacuoles associated with lipofuscin. Morphometric analysis showed that mitochondria are significantly reduced in Alzheimer's disease. The relationship shown here between the site and extent of mitochondrial abnormalities and oxidative damage suggests an intimate and early association between these features in Alzheimer's disease.
Figures



References
-
- Blass JP, Gibson GE. The role of oxidative abnormalities in the pathophysiology of Alzheimer's disease. Rev Neurol (Paris) 1991;147:513–525. - PubMed
-
- Blass JP, Baker AC, Ko L, Black RS. Induction of Alzheimer antigens by an uncoupler of oxidative phosphorylation. Arch Neurol. 1990;47:864–869. - PubMed
-
- Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275:395–403. - PubMed
-
- Dowson JH, Mountjoy CQ, Cairns MR, Wilton-Cox H, Bondareff W. Lipopigment changes in Purkinje cells in Alzheimer's disease. J Alzheimer's Dis. 1998;1:71–79. - PubMed
-
- Friguet B, Stadtman ER, Szweda LI. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Formation of cross-linked protein that inhibits the multicatalytic protease. J Biol Chem. 1994;269:21639–21643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
