Adaptive axonal remodeling in the midbrain auditory space map
- PMID: 11312301
- PMCID: PMC6762584
- DOI: 10.1523/JNEUROSCI.21-09-03161.2001
Adaptive axonal remodeling in the midbrain auditory space map
Abstract
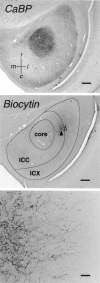
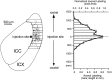
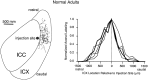
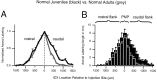
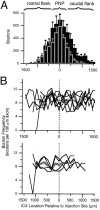
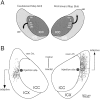
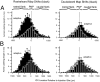
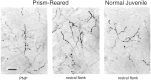
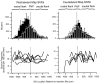
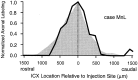
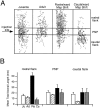
The auditory space map in the external nucleus of the inferior colliculus (ICX) of barn owls is highly plastic, especially during early life. When juvenile owls are reared with prismatic spectacles (prisms) that displace the visual field laterally, the auditory spatial tuning of neurons in the ICX adjusts adaptively to match the visual displacement. In the present study, we show that this functional plasticity is accompanied by axonal remodeling. The ICX receives auditory input from the central nucleus of the inferior colliculus (ICC) via topographic axonal projections. We used the anterograde tracer biocytin to study experience-dependent changes in the spatial pattern of axons projecting from the ICC to the ICX. The projection fields in normal adults were sparser and more restricted than those in normal juveniles. The projection fields in prism-reared adults were denser and broader than those in normal adults and contained substantially more bouton-laden axons that were appropriately positioned in the ICX to convey adaptive auditory spatial information. Quantitative comparison of results from juvenile and prism-reared owls indicated that prism experience led to topographically appropriate axonal sprouting and synaptogenesis. We conclude that this elaboration of axons represents the formation of an adaptive neuronal circuit. The density of axons and boutons in the normal projection zone was preserved in prism-reared owls. The coexistence of two different circuits encoding alternative maps of space may underlie the ability of prism-reared owls to readapt to normal conditions as adults.
Figures





References
-
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821. - PubMed
-
- Antonini A, Stryker MP. Plasticity of geniculocortical afferents after brief or prolonged monocular occlusion in the cat. J Comp Neurol. 1996;369:64–82. - PubMed
-
- Brickley SG, Keating MJ, Grant S. Experience-dependent mechanism of binocular map plasticity in Xenopus: incongruent connections are masked by retinal input. Neurosci Lett. 1994;182:13–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
