Receptor specificities of human respiroviruses
- PMID: 11312330
- PMCID: PMC114213
- DOI: 10.1128/JVI.75.10.4604-4613.2001
Receptor specificities of human respiroviruses
Abstract
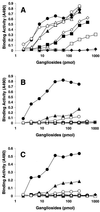
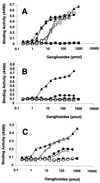
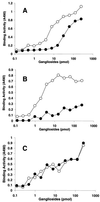
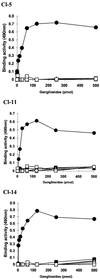
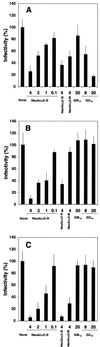
Through their hemagglutinin-neuraminidase glycoprotein, parainfluenza viruses bind to sialic acid-containing glycoconjugates to initiate infection. Although the virus-receptor interaction is a key factor of infection, the exact nature of the receptors that human parainfluenza viruses recognize has not been determined. We evaluated the abilities of human parainfluenza virus types 1 (hPIV-1) and 3 (hPIV-3) to bind to different types of gangliosides. Both hPIV-1 and hPIV-3 preferentially bound to neolacto-series gangliosides containing a terminal N-acetylneuraminic acid (NeuAc) linked to N-acetyllactosamine (Galbeta1-4GlcNAc) by the alpha2-3 linkage (NeuAcalpha2-3Galbeta1-4GlcNAc). Unlike hPIV-1, hPIV-3 bound to gangliosides with a terminal NeuAc linked to Galbeta1-4GlcNAc through an alpha2-6 linkage (NeuAcalpha2-6Galbeta1-4GlcNAc) or to gangliosides with a different sialic acid, N-glycolylneuraminic acid (NeuGc), linked to Galbeta1-4GlcNAc (NeuGcalpha2-3Galbeta1-4GlcNAc). These results indicate that the molecular species of glycoconjugate that hPIV-1 recognizes are more limited than those recognized by hPIV-3. Further analysis using purified gangliosides revealed that the oligosaccharide core structure is also an important element for binding. Gangliosides that contain branched N-acetyllactosaminoglycans in their core structure showed higher avidity than those without them. Agglutination of human, cow, and guinea pig erythrocytes but not equine erythrocytes by hPIV-1 and hPIV-3 correlated well with the presence or the absence of sialic acid-linked branched N-acetyllactosaminoglycans on the cell surface. Finally, NeuAcalpha2-3I, which bound to both viruses, inhibited virus infection of Lewis lung carcinoma-monkey kidney cells in a dose-dependent manner. We conclude that hPIV-1 and hPIV-3 preferentially recognize oligosaccharides containing branched N-acetyllactosaminoglycans with terminal NeuAcalpha2-3Gal as receptors and that hPIV-3 also recognizes NeuAcalpha2-6Gal- or NeuGcalpha2-3Gal-containing receptors. These findings provide important information that can be used to develop inhibitors that prevent human parainfluenza virus infection.
Figures

References
-
- Ah-Tye C, Schwartz S, Huberman K, Carlin E, Moscona A. Virus-receptor interactions of human parainfluenza viruses type 1, 2 and 3. Microb Pathog. 1999;27:329–336. - PubMed
-
- Baenziger J U, Fiete D. Structure determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:9795–9799. - PubMed
-
- Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1205–1241.
-
- Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. - PubMed
-
- Conyers S M, Kidwell D A. Chromogenic substrates for horseradish peroxidase. Anal Biochem. 1991;192:207–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

