Pregnenolone sulfate block of GABA(A) receptors: mechanism and involvement of a residue in the M2 region of the alpha subunit
- PMID: 11313438
- PMCID: PMC2278584
- DOI: 10.1111/j.1469-7793.2001.0673e.x
Pregnenolone sulfate block of GABA(A) receptors: mechanism and involvement of a residue in the M2 region of the alpha subunit
Abstract
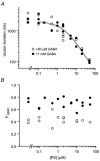
Neurosteroids are produced in the brain, and can have rapid actions on membrane channels of neurons. Pregnenolone sulfate (PS) is a sulfated neurosteroid which reduces the responses of the [gamma]-aminobutyric acid A (GABA(A)) receptor. We analysed the actions of PS on single-channel currents from recombinant GABA(A) receptors formed from [alpha]1, [beta]2 and [gamma]2L subunits. Currents were elicited by a concentration of GABA eliciting a half-maximal response (50 microM) and a saturating concentration (1 mM). PS reduced the duration of clusters of single-channel activity at either concentration of GABA. PS had no discernable effect on rapid processes: no effects were apparent on channel opening and closing, nor on GABA affinity, and a rapidly recovering desensitised state was not affected. Instead, PS produced a slowly developing block which occurred at a similar rate for receptors with open or closed channels and with one or two bound GABA molecules. The rate of block was independent of membrane potential, implying that the charged sulfate moiety does not move through the membrane field. Change in a specific residue near the intracellular end of the channel lining portion of the [alpha]1 subunit had a major effect on the rate of block. Mutation of the residue [alpha]1 V256S reduced the rate of block by 30-fold. A mutation at the homologous position of the [beta]2 subunit ([beta]2 A252S) had no effect, nor did a complementary mutation in the [gamma]2L subunit ([gamma]2L S266A). It seems likely that this residue is involved in a conformational change underlying block by PS, instead of forming part of the binding site for PS.
Figures

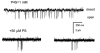



References
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. 2. New York: John Wiley; 1992.
-
- Charnet P, Labarca C, Leonard RJ, Vogelaar NJ, Czyzyk L, Gouin A, Davidson N, Lester HA. An open-channel blocker interacts with adjacent turns of α-helices in the nicotinic acetylcholine receptor. Neuron. 1990;4:87–95. - PubMed
-
- Corpechot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Research. 1997;766:276–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

