A bipartite yeast SSRP1 analog comprised of Pob3 and Nhp6 proteins modulates transcription
- PMID: 11313475
- PMCID: PMC100271
- DOI: 10.1128/MCB.21.10.3491-3502.2001
A bipartite yeast SSRP1 analog comprised of Pob3 and Nhp6 proteins modulates transcription
Abstract
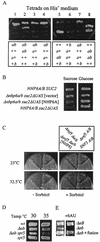
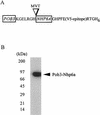
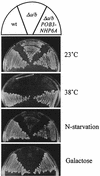
The FACT complex of vertebrate cells, comprising the Cdc68 (Spt16) and SSRP1 proteins, facilitates transcription elongation on a nucleosomal template and modulates the elongation-inhibitory effects of the DSIF complex in vitro. Genetic findings show that the related yeast (Saccharomyces cerevisiae) complex, termed CP, also mediates transcription. The CP components Cdc68 and Pob3 closely resemble the FACT components, except that the C-terminal high-mobility group (HMG) box domain of SSRP1 is not found in the yeast homolog Pob3. We show here that Nhp6a and Nhp6b, small HMG box proteins with overlapping functions in yeast, associate with the CP complex and mediate CP-related genetic effects on transcription. Absence of the Nhp6 proteins causes severe impairment in combination with mutations impairing the Swi-Snf chromatin-remodeling complex and the DSIF (Spt4 plus Spt5) elongation regulator, and sensitizes cells to 6-azauracil, characteristic of elongation effects. An artificial SSRP1-like protein, created by fusing the Pob3 and Nhp6a proteins, provides both Pob3 and Nhp6a functions for transcription, and competition experiments indicate that these functions are exerted in association with Cdc68. This particular Pob3-Nhp6a fusion protein was limited for certain Nhp6 activities, indicating that its Nhp6a function is compromised. These findings suggest that in yeast cells the Cdc68 partners may be both Pob3 and Nhp6, functioning as a bipartite analog of the vertebrate SSRP1 protein.
Figures


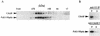
References
-
- Bortvin A, Winston F. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science. 1996;272:1473–1476. - PubMed
-
- Brewster N K, Johnston G C, Singer R A. Characterization of the CP complex, an abundant dimer of Cdc68 and Pob3 proteins that regulates yeast transcriptional activation and chromatin repression. J Biol Chem. 1998;273:21972–21979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
