Activated PAK4 regulates cell adhesion and anchorage-independent growth
- PMID: 11313478
- PMCID: PMC100274
- DOI: 10.1128/MCB.21.10.3523-3533.2001
Activated PAK4 regulates cell adhesion and anchorage-independent growth
Abstract
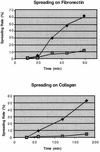
The serine/threonine kinase PAK4 is an effector molecule for the Rho GTPase Cdc42. PAK4 differs from other members of the PAK family in both sequence and function. Previously we have shown that an important function of this kinase is to mediate the induction of filopodia in response to activated Cdc42. Since previous characterization of PAK4 was carried out only with the wild-type kinase, we have generated a constitutively active mutant of the kinase to determine whether it has other functions. Expression of activated PAK4 in fibroblasts led to a transient induction of filopodia, which is consistent with its role as an effector for Cdc42. In addition, use of the activated mutant revealed a number of other important functions of this kinase that were not revealed by studying the wild-type kinase. For example, activated PAK4 led to the dissolution of stress fibers and loss of focal adhesions. Consequently, cells expressing activated PAK4 had a defect in cell spreading onto fibronectin-coated surfaces. Most importantly, fibroblasts expressing activated PAK4 had a morphology that was characteristic of oncogenic transformation. These cells were anchorage independent and formed colonies in soft agar, similar to what has been observed previously in cells expressing activated Cdc42. Consistent with this, dominant-negative PAK4 mutants inhibited focus formation by oncogenic Dbl, an exchange factor for Rho family GTPases. These results provide the first demonstration that a PAK family member can transform cells and indicate that PAK4 may play an essential role in oncogenic transformation by the GTPases. We propose that the morphological changes and changes in cell adhesion induced by PAK4 may play a direct role in oncogenic transformation by Rho family GTPases and their exchange factors.
Figures








References
-
- Bagrodia S, Cerione R A. PAK to the future. Trends Cell Biol. 1999;9:350–355. - PubMed
-
- Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. - PubMed
-
- Bagrodia S, Taylor S J, Creasy C L, Chernoff J, Cerione R A. Identification of a mouse p21 Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–22737. - PubMed
-
- Brown J L, Stowers L, Baer M, Trejo J, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
