Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX
- PMID: 11313479
- PMCID: PMC100275
- DOI: 10.1128/MCB.21.10.3534-3546.2001
Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX
Abstract
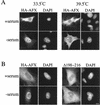
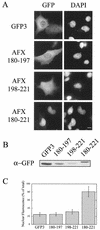
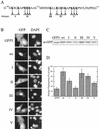
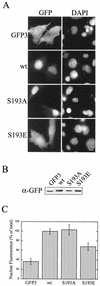
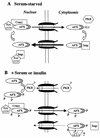
AFX belongs to a subfamily of Forkhead transcription factors that are phosphorylated by protein kinase B (PKB), also known as Akt. Phosphorylation inhibits the transcriptional activity of AFX and changes the steady-state localization of the protein from the nucleus to the cytoplasm. Our goal was threefold: to identify the cellular compartment in which PKB phosphorylates AFX, to determine whether the nuclear localization of AFX plays a role in regulating its transcriptional activity, and to elucidate the mechanism by which phosphorylation alters the localization of AFX. We show that phosphorylation of AFX by PKB occurs in the nucleus. In addition, nuclear export mediated by the export receptor, Crm1, is required for the inhibition of AFX transcriptional activity. Both phosphorylated and unphosphorylated AFX, however, bind Crm1 and can be exported from the nucleus. These results suggest that export is unregulated and that phosphorylation by PKB is not required for the nuclear export of AFX. We show that AFX enters the nucleus by an active, Ran-dependent mechanism. Amino acids 180 to 221 of AFX comprise a nonclassical nuclear localization signal (NLS). S193, contained within this atypical NLS, is a PKB-dependent phosphorylation site on AFX. Addition of a negative charge at S193 by mutating the residue to glutamate reduces nuclear accumulation. PKB-mediated phosphorylation of AFX, therefore, attenuates the import of the transcription factor, which shifts the localization of the protein from the nucleus to the cytoplasm and results in the inhibition of AFX transcriptional activity.
Figures



References
-
- Ahmed N N, Franke T F, Bellacosa A, Datta K, Gonzalez-Portal M E, Taguchi T, Testa J R, Tsichlis P N. The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene. 1993;8:1957–1963. - PubMed
-
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B α. Curr Biol. 1997;7:261–269. - PubMed
-
- Anderson M J, Viars C S, Czekay S, Cavenee W K, Arden K C. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. - PubMed
-
- Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
-
- Arts G J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
