Purification of Synechocystis sp. strain PCC6308 cyanophycin synthetase and its characterization with respect to substrate and primer specificity
- PMID: 11319097
- PMCID: PMC92852
- DOI: 10.1128/AEM.67.5.2176-2182.2001
Purification of Synechocystis sp. strain PCC6308 cyanophycin synthetase and its characterization with respect to substrate and primer specificity
Abstract
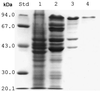
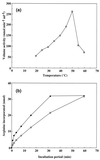
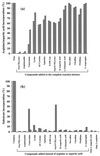
Synechocystis sp. strain PCC6308 cyanophycin synthetase was purified 72-fold in three steps by anion exchange chromatography on Q Sepharose, affinity chromatography on the triazine dye matrix Procion Blue HE-RD Sepharose, and gel filtration on Superdex 200 HR from recombinant cells of Escherichia coli. The native enzyme, which catalyzed the incorporation of arginine and aspartic acid into cyanophycin, has an apparent molecular mass of 240 +/- 30 kDa and consists of identical subunits of 85 +/- 5 kDa. The K(m) values for arginine (49 microM), aspartic acid (0.45 mM), and ATP (0.20 mM) indicated that the enzyme had a high affinity towards these substrates. During in vitro cyanophycin synthesis, 1.3 +/- 0.1 mol of ATP per mol of incorporated amino acid was converted to ADP. The optima for the enzyme-catalyzed reactions were pH 8.2 and 50 degrees C, respectively. Arginine methyl ester (99.5 and 97% inhibition), argininamide (99 and 96%), S-(2-aminoethyl) cysteine (43 and 42%), beta-hydroxy aspartic acid (35 and 37%), aspartic acid beta-methyl ester (38 and 40%), norvaline (0 and 3%), citrulline (9 and 7%), and asparagine (2 and 0%) exhibited an almost equal inhibitory effect on the incorporation of both arginine and aspartic acid, respectively, when these compounds were added to the complete reaction mixture. In contrast, the incorporation of arginine was diminished to a greater extent than that of aspartic acid, respectively, with canavanine (82 and 53%), lysine (36 and 19%), agmatine (33 and 25%), D-aspartic acid (37 and 30%), L-glutamic acid (13 and 5%), and ornithine (23 and 11%). On the other hand, canavanine (45% of maximum activity) and lysine (13%) stimulated the incorporation of aspartic acid, whereas aspartic acid beta-methyl ester (53%) and asparagine (9%) stimulated the incorporation of arginine. [(3)H]lysine (15% of maximum activity) and [(3)H]canavanine (13%) were incorporated into the polymer, when they were either used instead of arginine or added to the complete reaction mixture, whereas L-glutamic acid was not incorporated. No effect on arginine incorporation was obtained by the addition of other amino acids (i.e., alanine, histidine, leucine, proline, tryptophan, and glycine). Various samples of chemically synthesized poly-alpha,beta-D,L-aspartic acid served as primers for in vitro synthesis of cyanophycin, whereas poly-alpha-L-aspartic acid was almost inactive.
Figures


References
-
- Aboulmagd E, Oppermann-Sanio F B, Steinbüchel A. Molecular characterization of the cyanophycin synthetase from Synechocystissp. strain PCC6308. Arch Microbiol. 2000;174:297–306. - PubMed
-
- Allen M M. Cyanobacterial cell inclusions. Annu Rev Microbiol. 1984;38:1–25. - PubMed
-
- Atkinson T, Hammond P M, Hartwell R D, Hughes P, Scaven M D, Sherwood R F, Small A P, Bruton C J, Harvey M J, Lowe C R. Triazine-dye affinity chromatography. Biochem Soc Trans. 1981;9:290–293. - PubMed
-
- Berg H, Ziegler K, Piotukh K, Baier K, Lockau W, Volker-Engert R. Biosynthesis of the cyanobacterial reserve polymer multi-l-arginyl-poly-l-aspartic acid (cyanophycin). Mechanism of the cyanophycin synthetase reaction studied with synthetic primers. Eur J Biochem. 2000;267:5561–5570. - PubMed
-
- Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

