BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana
- PMID: 11320207
- PMCID: PMC33313
- DOI: 10.1073/pnas.091065998
BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana
Abstract
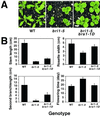
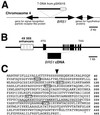
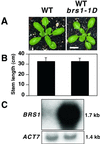
Brassinosteroid-insensitive 1 (BRI1) of Arabidopsis thaliana encodes a cell surface receptor for brassinosteroids. Mutations in BRI1 severely affect plant growth and development. Activation tagging of a weak bri1 allele (bri1-5) resulted in the identification of a new locus, brs1-1D. BRS1 is predicted to encode a secreted carboxypeptidase. Whereas a brs1 loss-of-function allele has no obvious mutant phenotype, overexpression of BRS1 can suppress bri1 extracellular domain mutants. Genetic analyses showed that brassinosteroids and a functional BRI1 protein kinase domain are required for suppression. In addition, overexpressed BRS1 missense mutants, predicted to abolish BRS1 protease activity, failed to suppress bri1-5. Finally, the effects of BRS1 are selective: overexpression in either wild-type or two other receptor kinase mutants resulted in no phenotypic alterations. These results strongly suggest that BRS1 processes a protein involved in an early event in the BRI1 signaling.
Figures



References
-
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Plant J. 1996;9:701–713.
-
- Li J, Chory J. Cell. 1997;90:929–938. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

