Structure and function in rhodopsin: Mass spectrometric identification of the abnormal intradiscal disulfide bond in misfolded retinitis pigmentosa mutants
- PMID: 11320236
- PMCID: PMC33130
- DOI: 10.1073/pnas.061632798
Structure and function in rhodopsin: Mass spectrometric identification of the abnormal intradiscal disulfide bond in misfolded retinitis pigmentosa mutants
Abstract
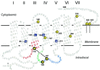
Retinitis pigmentosa (RP) point mutations in both the intradiscal (ID) and transmembrane domains of rhodopsin cause partial or complete misfolding of rhodopsin, resulting in loss of 11-cis-retinal binding. Previous work has shown that misfolding is caused by the formation of a disulfide bond in the ID domain different from the native Cys-110-Cys-187 disulfide bond in native rhodopsin. Here we report on direct identification of the abnormal disulfide bond in misfolded RP mutants in the transmembrane domain by mass spectrometric analysis. This disulfide bond is between Cys-185 and Cys-187, the same as previously identified in misfolded RP mutations in the ID domain. The strategy described here should be generally applicable to identification of disulfide bonds in other integral membrane proteins.
Figures

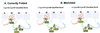

Comment in
-
How activated receptors couple to G proteins.Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):4819-21. doi: 10.1073/pnas.011099798. Proc Natl Acad Sci U S A. 2001. PMID: 11320227 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

