Solution 19F nuclear Overhauser effects in structural studies of the cytoplasmic domain of mammalian rhodopsin
- PMID: 11320239
- PMCID: PMC33133
- DOI: 10.1073/pnas.051633098
Solution 19F nuclear Overhauser effects in structural studies of the cytoplasmic domain of mammalian rhodopsin
Abstract
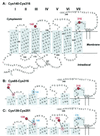
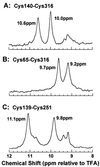
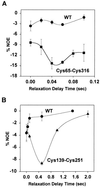
19F nuclear Overhauser effects (NOEs) between fluorine labels on the cytoplasmic domain of rhodopsin solubilized in detergent micelles are reported. Previously, high-resolution solution (19)F NMR spectra of fluorine-labeled rhodopsin in detergent micelles were described, demonstrating the applicability of this technique to studies of tertiary structure in the cytoplasmic domain. To quantitate tertiary contacts we have applied a transient one-dimensional difference NOE solution (19)F NMR experiment to this system, permitting assessment of proximities between fluorine labels specifically incorporated into different regions of the cytoplasmic face. Three dicysteine substitution mutants (Cys-140-Cys-316, Cys-65-Cys-316, and Cys-139-Cys-251) were labeled by attachment of the trifluoroethylthio group through a disulfide linkage. Each mutant rhodopsin was prepared (8-10 mg) in dodecylmaltoside and analyzed at 20 degrees C by solution (19)F NMR. Distinct chemical shifts were observed for all of the rhodopsin (19)F labels in the dark. An up-field shift of the Cys-316 resonance in the Cys-65-Cys-316 mutant suggests a close proximity between the two residues. When analyzed for (19)F-(19)F NOEs, a moderate negative enhancement was observed for the Cys-65-Cys-316 pair and a strong negative enhancement was observed for the Cys-139-Cys-251 pair, indicating proximity between these sites. No NOE enhancement was observed for the Cys-140-Cys-316 pair. These NOE effects demonstrate a solution (19)F NMR method for analysis of tertiary contacts in high molecular weight proteins, including membrane proteins.
Figures
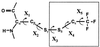

Comment in
-
How activated receptors couple to G proteins.Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):4819-21. doi: 10.1073/pnas.011099798. Proc Natl Acad Sci U S A. 2001. PMID: 11320227 Free PMC article. No abstract available.
References
-
- Cai K, Klein-Seetharaman J, Farrens D, Zhang C, Altenbach C, Hubbell W L, Khorana H G. Biochemistry. 1999;38:7925–7930. - PubMed
-
- Altenbach C, Cai K, Khorana H G, Hubbell W L. Biochemistry. 1999;38:7931–7937. - PubMed
-
- Altenbach C, Klein-Seetharaman J, Hwa J, Khorana H G, Hubbell W L. Biochemistry. 1999;38:7945–7949. - PubMed
-
- Klein-Seetharaman J, Hwa J, Cai K, Altenbach C, Hubbell W L, Khorana H G. Biochemistry. 1999;38:7938–7944. - PubMed
-
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox B A, Le Trong I, Teller D C, Okada T, Stenkamp R E. Science. 2000;289:739–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

