Supercharged protein and peptide ions formed by electrospray ionization
- PMID: 11321294
- PMCID: PMC1414801
- DOI: 10.1021/ac001251t
Supercharged protein and peptide ions formed by electrospray ionization
Abstract
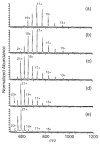
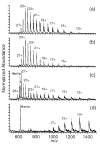
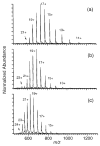
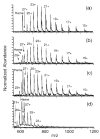
The multiple charging of large molecules in electrospray ionization provides key advantages for obtaining accurate molecular weights by mass spectrometry and for obtaining structural information by tandem mass spectrometry and MS(n) experiments. Addition of glycerol or m-nitrobenzyl alcohol into the electrospray solutions dramatically increases both the maximum observed charge state and the abundances of the high charge states of protein and peptide ions. Adding glycerol to acidified aqueous solutions of cytochrome c shifts the most abundant charge state from 17+ to 21+, shifts the maximum charge state from 20+ to 23+, and shifts the average charge state from 16.6+ to 20.9+. Much less m-nitrobenzyl alcohol (<1%) is required to produce similar results. With just 0.7% m-nitrobenzyl alcohol, even the 24+ charge state of cytochrome c is readily observed. Similar results are obtained with myoglobin and (Lys)4. For the latter molecule, the 5+ charge state is observed in the electrospray mass spectrum obtained from solutions containing 6.7% m-nitrobenzyl alcohol. This charge state corresponds to protonation of all basic sites in this peptide. Although the mechanism for enhanced charging is unclear, it does not appear to be a consequence of conformational changes of the analyte molecules. This method of producing highly charged protein ions should be useful for improving the performance of mass measurements on mass spectrometers with performances that decrease with increasing m/z. This should also be particularly useful for tandem mass spectrometry experiments, such as electron capture dissociation, for which highly charged ions are desired.
Figures

References
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. - PubMed
-
- Hillenkamp F, Karas M, Beavis RC, Chait BT. Anal Chem. 1991;63:1193A–1202A. - PubMed
-
- Veenstra TD. Biophys Chem. 1999;79:63–79. and references therein. - PubMed
-
- Chen R, Cheng X, Mitchell DW, Hofstadler SA, Wu Q, Rockwood AL, Sherman MG, Smith RD. Anal Chem. 1995;67:1159–1163.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

