Involvement of accumulated endogenous NOS inhibitors and decreased NOS activity in the impaired neurogenic relaxation of the rabbit proximal urethra with ischaemia
- PMID: 11325799
- PMCID: PMC1572766
- DOI: 10.1038/sj.bjp.0704050
Involvement of accumulated endogenous NOS inhibitors and decreased NOS activity in the impaired neurogenic relaxation of the rabbit proximal urethra with ischaemia
Abstract
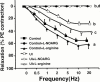
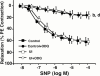
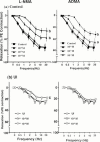
1. We examined the effect of ischaemia on the neurogenic and nitric oxide (NO)-mediated urethral relaxation. 2. Rabbits were divided into control and urethral ischaemia (UI) groups, which was prepared by the partial occlusion of bilateral iliac arteries using blood vessel occluders. 3. Neurogenic and NO-mediated proximal urethral relaxation induced by electrical field stimulation (EFS) was greatly impaired in the UI group, while relaxation by sodium nitroprusside (SNP) as a NO donor showed no difference between the two groups. Pretreatment with L-arginine significantly improved but did not normalize the impaired relaxation in the UI group. Not only basal level, but also stimulated production of cyclic GMP with EFS, were significantly decreased in the UI group. 4. The tissue contents of N(G)-methyl-L-arginine (L-NMA) and asymmetric N(G), N(G)-dimethyl-L-arginine (ADMA) in the proximal urethra were increased following ischaemia. While L-arginine and symmetric N(G), N'(G)-dimethyl-L-arginine (SDMA) contents remained unchanged. Exogenously applied authentic L-NMA and ADMA (1 -- 100 microM) concentration-dependently inhibited the EFS-induced urethral relaxation in the control group. The inhibition with L-NMA and ADMA was undetectable in the presence of 3 mM L-arginine. 5. The Ca(2+)-dependent NOS activity in the urethra from the UI group was significantly lower than that from the control group and was not restored by an addition of 3 mM L-arginine. 6. These results suggest that the impaired neurogenic and NO-mediated urethral relaxation with ischaemia is closely related to the increased accumulation of L-NMA and ADMA and decreased NOS activity, which would result in an accelerated reduction in NO production/release.
Figures





References
-
- ANDERSSON K.-E., GARCIA-PASCUAL A., PERSSON K., FORMAN A., TOTTRUP A. Electrically-induced nerve-mediated relaxation of rabbit urethra involves nitric oxide. J. Urol. 1992;147:253–259. - PubMed
-
- AZADZOI K.M., GOLDSTEIN I., SIROKY M.B., TRAISH A.M., KRANE R.B., SAENZ DE TEJADA I. Mechanisms of ischemia-induced cavernosal smooth muscle relaxation impairment in a rabbit model of vasculogenic erectile dysfunction. J. Urol. 1998;160:2216–2222. - PubMed
-
- AZUMA H., MASUDA H., SATO J., NIWA K., TOKORO T. A possible role of endogenous inhibitor for nitric oxide synthesis in the bovine ciliary muscle. Exp. Eye Res. 1997;64:823–830. - PubMed
-
- BENNETT B.C., KRUSE M.N., ROPPOLO J.R., FLOOD H.D., FRASER M., DE GROAT W.C. Neural control of urethral outlet activity in vivo: role of nitric oxide. J. Urol. 1995;153:2004–2009. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

