The properties of ryanodine-sensitive Ca(2+) release in mouse gastric smooth muscle cells
- PMID: 11325802
- PMCID: PMC1572764
- DOI: 10.1038/sj.bjp.0704048
The properties of ryanodine-sensitive Ca(2+) release in mouse gastric smooth muscle cells
Abstract
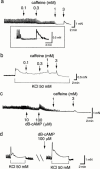
1. Under voltage-clamped conditions, gastric smooth muscle cells of BALB/c mice developed spontaneous (STOCs) and caffeine- (I(CAF)) and carbachol-induced (I(CCh)) transient outward currents. 2. In fura-2 microscopic measurements of intracellular Ca(2+) concentration ([Ca(2+)](i)), caffeine and carbachol (CCh) provoked similar transient [Ca(2+)](i) elevations. 3. Both I(CCh) and CCh-induced [Ca(2+)](i) elevation of single smooth muscle cells occurred in an 'all-or-nothing' fashion in contrast to the reproducible caffeine responses. 4. On the basis of the suppression of STOCs and I(CAF) by nicardipine, tetraethylammonium and iberiotoxin, but not by charybdotoxin nor apamin, it was suggested that both currents were generated by large conductance type Ca(2+)-activated K(+) channels. 5. In measurements of isometric tension, caffeine produced relaxation of gastric smooth muscle strips in a concentration-dependent manner (0.1 -- 3 mM). The concentration-dependent relaxation with caffeine was mimicked by dibutyryl cyclic AMP which produced potentiation of contraction triggered by 50 mM KCL. 6. At caffeine concentrations >3 mM, a transient contraction followed by relaxation was provoked as the quasi maximal response to caffeine. In the quasi maximal response, caffeine acted as a potent relaxant in smooth muscle strips precontracted with 50 mM KCl or 3 microM CCh. 7. The relaxation with caffeine was significantly accelerated in those strips precontracted with KCl or CCh. All these results suggest that ryanodine-sensitive Ca(2+) release, which is triggered by caffeine, is an important modifier of Ca(2+) homeostasis in the cytoplasm and the contractility of gastric smooth muscle cells of mice.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

