The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin
- PMID: 11325939
- PMCID: PMC95211
- DOI: 10.1128/JB.183.10.3108-3116.2001
The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin
Abstract
It was previously proposed that autolysin's primary role in the virulence of pneumococci was to release pneumolysin to an extracellular location. This interpretation came into question when pneumolysin was observed to be released in significant amounts from some pneumococci during log-phase growth, because autolysis was not believed to occur at this time. We have reexamined this phenomenon in detail for one such strain, WU2. This study found that the extracellular release of pneumolysin from WU2 was not dependent on autolysin action. A mutant lacking autolysin showed the same pattern of pneumolysin release as the wild-type strain. Addition of mitomycin C to a growing WU2 culture did not induce lysis, indicating the absence of resident bacteriophages that could potentially harbor lytA-like genes. Furthermore, release of pneumolysin was unaltered by growth in 2% choline, a condition which is reported to inactivate autolysin, as well as most known pneumococcal phage lysins. Profiles of total proteins in the cytoplasm and in the supernatant media supported the hypothesis that release of pneumolysin is independent of pneumococcal lysis. Finally, under some infection conditions, mutations in pneumolysin and autolysin had different effects on virulence.
Figures





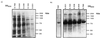

References
-
- Berry A M, Paton J C, Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992;12:87–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

