CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans
- PMID: 11325951
- PMCID: PMC95223
- DOI: 10.1128/JB.183.10.3211-3223.2001
CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans
Abstract
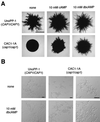
In response to a wide variety of environmental stimuli, the opportunistic fungal pathogen Candida albicans exits the budding cycle, producing germ tubes and hyphae concomitant with expression of virulence genes, such as that encoding hyphal wall protein 1 (HWP1). Biochemical studies implicate cyclic AMP (cAMP) increases in promoting bud-hypha transitions, but genetic evidence relating genes that control cAMP levels to bud-hypha transitions has not been reported. Adenylate cyclase-associated proteins (CAPs) of nonpathogenic fungi interact with Ras and adenylate cyclase to increase cAMP levels under specific environmental conditions. To initiate studies on the relationship between cAMP signaling and bud-hypha transitions in C. albicans, we identified, cloned, characterized, and disrupted the C. albicans CAP1 gene. C. albicans strains with inactivated CAP1 budded in conditions that led to germ tube formation in isogenic strains with CAP1. The addition of 10 mM cAMP and dibutyryl cAMP promoted bud-hypha transitions and filamentous growth in the cap1/cap1 mutant in liquid and solid media, respectively, showing clearly that cAMP promotes hypha formation in C. albicans. Increases in cytoplasmic cAMP preceding germ tube emergence in strains having CAP1 were markedly diminished in the budding cap1/cap1 mutant. C. albicans strains with deletions of both alleles of CAP1 were avirulent in a mouse model of systemic candidiasis. The avirulence of a germ tube-deficient cap1/cap1 mutant coupled with the role of Cap1 in regulating cAMP levels shows that the Cap1-mediated cAMP signaling pathway is required for bud-hypha transitions, filamentous growth, and the pathogenesis of candidiasis.
Figures





References
-
- Anderson J M, Soll D R. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J Gen Microbiol. 1986;132:2035–2047. - PubMed
-
- Barlow A J, Aldersley T, Chattaway F W. Factors present in serum and seminal plasma which promote germ-tube formation and mycelial growth of Candida albicans. J Gen Microbiol. 1974;82:261–272. - PubMed
-
- Baum B, Li W, Perrimon N. A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr Biol. 2000;10:964–973. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

