Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death
- PMID: 11326099
- PMCID: PMC3049805
- DOI: 10.1126/science.1059108
Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death
Abstract
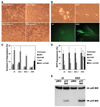
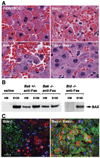
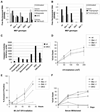
Multiple death signals influence mitochondria during apoptosis, yet the critical initiating event for mitochondrial dysfunction in vivo has been unclear. tBID, the caspase-activated form of a "BH3-domain-only" BCL-2 family member, triggers the homooligomerization of "multidomain" conserved proapoptotic family members BAK or BAX, resulting in the release of cytochrome c from mitochondria. We find that cells lacking both Bax and Bak, but not cells lacking only one of these components, are completely resistant to tBID-induced cytochrome c release and apoptosis. Moreover, doubly deficient cells are resistant to multiple apoptotic stimuli that act through disruption of mitochondrial function: staurosporine, ultraviolet radiation, growth factor deprivation, etoposide, and the endoplasmic reticulum stress stimuli thapsigargin and tunicamycin. Thus, activation of a "multidomain" proapoptotic member, BAX or BAK, appears to be an essential gateway to mitochondrial dysfunction required for cell death in response to diverse stimuli.
Figures

Comment in
-
The mitochondrion: is it central to apoptosis?Science. 2001 Apr 27;292(5517):624-6. doi: 10.1126/science.292.5517.624. Science. 2001. PMID: 11330312 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

