Flagellin polymerisation control by a cytosolic export chaperone
- PMID: 11327763
- PMCID: PMC2528291
- DOI: 10.1006/jmbi.2001.4597
Flagellin polymerisation control by a cytosolic export chaperone
Abstract
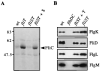
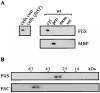
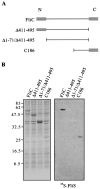
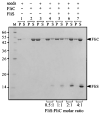
Assembly of the long helical filament of the bacterial flagellum requires polymerisation of ca 20,000 flagellin (FliC) monomeric subunits into the growing structure extending from the cell surface. Here, we show that export of Salmonella flagellin is facilitated specifically by a cytosolic protein, FliS, and that FliS binds to the FliC C-terminal helical domain, which contributes to stabilisation of flagellin subunit interactions during polymerisation. Stable complexes of FliS with flagellin were assembled efficiently in vitro, apparently by FliS homodimers binding to FliC monomers. The data suggest that FliS acts as a substrate-specific chaperone, preventing premature interaction of newly synthesised flagellin subunits in the cytosol. Compatible with this view, FliS was able to prevent in vitro polymerisation of FliC into filaments.
Copyright 2001 Academic Press.
Figures



References
-
- O’Brien E, Bennett PM. Structure of straight flagella from a mutant Salmonella. J. Mol. Biol. 1972;70:133–152. - PubMed
-
- Aizawa S-I. Flagellar assembly in Salmonella typhimurium. Mol. Microbiol. 1996;19:1–5. - PubMed
-
- Macnab RM. Flagella and motility. In: Neidhardt FC, editor. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. 2nd edit. Washington, DC: American Society for Microbiology; 1996. pp. 123–145.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

