Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease
- PMID: 11328868
- PMCID: PMC37250
- DOI: 10.1093/nar/29.9.1852
Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease
Abstract
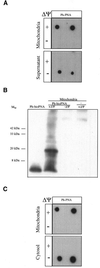
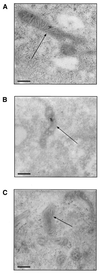
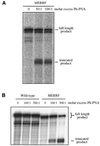
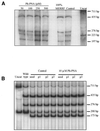
The selective manipulation of mitochondrial DNA (mtDNA) replication and expression within mammalian cells has proven difficult. One promising approach is to use peptide nucleic acid (PNA) oligomers, nucleic acid analogues that bind selectively to complementary DNA or RNA sequences inhibiting replication and translation. However, the potential of PNAs is restricted by the difficulties of delivering them to mitochondria within cells. To overcome this problem we conjugated a PNA 11mer to a lipophilic phosphonium cation. Such cations are taken up by mitochondria through the lipid bilayer driven by the membrane potential across the inner membrane. As anticipated, phosphonium-PNA (ph-PNA) conjugates of 3.4-4 kDa were imported into both isolated mitochondria and mitochondria within human cells in culture. This was confirmed by using an ion-selective electrode to measure uptake of the ph-PNA conjugates; by cell fractionation in conjunction with immunoblotting; by confocal microscopy; by immunogold-electron microscopy; and by crosslinking ph-PNA conjugates to mitochondrial matrix proteins. In all cases dissipating the mitochondrial membrane potential with an uncoupler prevented ph-PNA uptake. The ph-PNA conjugate selectively inhibited the in vitro replication of DNA containing the A8344G point mutation that causes the human mtDNA disease 'myoclonic epilepsy and ragged red fibres' (MERRF) but not the wild-type sequence that differs at a single nucleotide position. Therefore these modified PNA oligomers retain their selective binding to DNA and the lipophilic cation delivers them to mitochondria within cells. When MERRF cells were incubated with the ph-PNA conjugate the ratio of MERRF to wild-type mtDNA was unaffected, even though the ph-PNA content of the mitochondria was sufficient to inhibit MERRF mtDNA replication in a cell-free system. This unexpected finding suggests that nucleic acid derivatives cannot bind their complementary sequences during mtDNA replication. In summary, we have developed a new strategy for targeting PNA oligomers to mitochondria and used it to determine the effects of PNA on mutated mtDNA replication in cells. This work presents new approaches for the manipulation of mtDNA replication and expression, and will assist in the development of therapies for mtDNA diseases.
Figures




References
-
- Larsson N.G. and Clayton,D.A. (1995) Molecular genetic aspects of human mitochondrial disorders. Annu. Rev. Genet., 29, 151–178. - PubMed
-
- Taylor R.W., Chinnery,P.F., Clark,K.M., Lightowlers,R.N. and Turnbull,D.M. (1997) Treatment of mitochondrial disease. J. Bioenerg. Biomembr., 29, 195–205. - PubMed
-
- Chrzanowska-Lightowlers L.Z., Lightowlers,R.N. and Turnbull,D.M. (1995) Gene therapy for mitochondrial DNA defects: is it possible? Gene Ther., 2, 311–316. - PubMed
-
- Clayton D.A. (1991) Nuclear gadgets in mitochondrial DNA replication and transcription. Trends Biochem. Sci., 16, 107–111. - PubMed
-
- Agrawal S. (1996) Antisense therapeutics. In Walker,J.M., (ed.), Methods in Molecular Medicine. Humana Press, NJ.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

