Importance of complement source in measuring meningococcal bactericidal titers
- PMID: 11329468
- PMCID: PMC96111
- DOI: 10.1128/CDLI.8.3.616-623.2001
Importance of complement source in measuring meningococcal bactericidal titers
Abstract
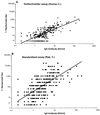
Complement-mediated bactericidal antibodies in serum confer protection against meningococcal disease. The minimum protective titer is estimated to be between 1:4 and 1:8 when measured by the Goldschneider assay performed with human complement, the assay used in the 1960s to establish the correlation between bactericidal antibodies and protection. A more recently described bactericidal assay standardized by an international consortium uses rabbit complement, which is known to augment bactericidal titers. To define a protective titer measured by the standardized assay, we compared bactericidal titers against serogroup C strains measured by this assay to titers measured by the assay described by Goldschneider et al. A titer of > or =1:128 measured by the standardized assay was needed to predict with > or =80% certainty a positive titer of > or =1:4 as measured by the Goldschneider assay. However, the majority of samples with titers of 1:4 measured by the Goldschneider assay had titers of <1:128 when measured by the standardized assay. Therefore, by the results of the standardized assay such persons would be falsely categorized as being susceptible to disease. In conclusion, high bactericidal titers measured with the standardized assay performed with rabbit complement are predictive of protection, but no threshold titer is both sensitive and specific for predicting a positive titer measured by the Goldschneider assay using human complement. Up to 10% of the U.S. adult population lacks intrinsic bactericidal activity against serogroup C strains in serum and can serve as complement donors. Therefore, use of the Goldschneider assay or an equivalent assay performed with human complement is preferred over assays that use rabbit complement.
Figures



References
-
- de Kleijn E D, de Groot R, Labadie J, Lafeber A B, van den Dobbelsteen G, van Alphen L, van Dijken H, Kuipers B, van Omme G W, Wala M, Juttmann R, Rumke H C. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2–3 and 7–8 years of age. Vaccine. 2000;18:1456–1466. - PubMed
-
- Edwards E A, Devine L F, Sengbusch G H, Ward H W. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis. 1977;9:105–110. - PubMed
-
- Frasch C E. Meningococcal vaccines: past, present and future. In: Cartwright K, editor. Meningococcal disease. New York, N.Y: John Wiley & Sons; 1995. pp. 245–283.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

