Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization
- PMID: 11331300
- PMCID: PMC2190570
- DOI: 10.1083/jcb.153.3.479
Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization
Abstract
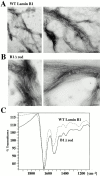
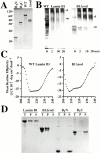
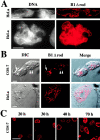
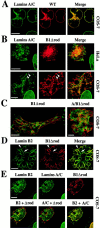
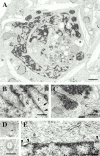
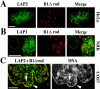
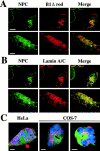
The nuclear lamina is a meshwork of intermediate-type filament proteins (lamins) that lines the inner nuclear membrane. The lamina is proposed to be an important determinant of nuclear structure, but there has been little direct testing of this idea. To investigate lamina functions, we have characterized a novel lamin B1 mutant lacking the middle approximately 4/5 of its alpha-helical rod domain. Though retaining only 10 heptads of the rod, this mutant assembles into intermediate filament-like structures in vitro. When expressed in cultured cells, it concentrates in patches at the nuclear envelope. Concurrently, endogenous lamins shift from a uniform to a patchy distribution and lose their complete colocalization, and nuclei become highly lobulated. In vitro binding studies suggest that the internal rod region is important for heterotypic associations of lamin B1, which in turn are required for proper organization of the lamina. Accompanying the changes in lamina structure induced by expression of the mutant, nuclear pore complexes and integral membrane proteins of the inner membrane cluster, principally at the patches of endogenous lamins. Considered together, these data indicate that lamins play a major role in organizing other proteins in the nuclear envelope and in determining nuclear shape.
Figures

Similar articles
-
The role of CaaX-dependent modifications in membrane association of Xenopus nuclear lamin B3 during meiosis and the fate of B3 in transfected mitotic cells.J Cell Biol. 1993 Dec;123(6 Pt 2):1661-70. doi: 10.1083/jcb.123.6.1661. J Cell Biol. 1993. PMID: 8276888 Free PMC article.
-
Lamins: The backbone of the nucleocytoskeleton interface.Curr Opin Cell Biol. 2024 Feb;86:102313. doi: 10.1016/j.ceb.2023.102313. Epub 2024 Jan 22. Curr Opin Cell Biol. 2024. PMID: 38262116 Review.
-
Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection.J Virol. 2001 Sep;75(18):8818-30. doi: 10.1128/jvi.75.18.8818-8830.2001. J Virol. 2001. PMID: 11507226 Free PMC article.
-
Investigations of the pathway of incorporation and function of lamin A in the nuclear lamina.Microsc Res Tech. 1999 Apr 1;45(1):1-12. doi: 10.1002/(SICI)1097-0029(19990401)45:1<1::AID-JEMT1>3.0.CO;2-Z. Microsc Res Tech. 1999. PMID: 10206150
-
Nuclear lamina at the crossroads of the cytoplasm and nucleus.J Struct Biol. 2012 Jan;177(1):24-31. doi: 10.1016/j.jsb.2011.11.007. Epub 2011 Nov 22. J Struct Biol. 2012. PMID: 22126840 Free PMC article. Review.
Cited by
-
Slower diffusion and anomalous association of R453W lamin A protein alter nuclear architecture in AD-EDMD.RSC Adv. 2022 Nov 9;12(49):32129-32141. doi: 10.1039/d2ra05620h. eCollection 2022 Nov 3. RSC Adv. 2022. PMID: 36415558 Free PMC article.
-
Novel nuclear herniations induced by nuclear localization of a viral protein.J Virol. 2004 Jun;78(12):6360-9. doi: 10.1128/JVI.78.12.6360-6369.2004. J Virol. 2004. PMID: 15163729 Free PMC article.
-
Concentration-dependent Effects of Nuclear Lamins on Nuclear Size in Xenopus and Mammalian Cells.J Biol Chem. 2015 Nov 13;290(46):27557-71. doi: 10.1074/jbc.M115.673798. Epub 2015 Oct 1. J Biol Chem. 2015. PMID: 26429910 Free PMC article.
-
Cell-specific and lamin-dependent targeting of novel transmembrane proteins in the nuclear envelope.Cell Mol Life Sci. 2010 Apr;67(8):1353-69. doi: 10.1007/s00018-010-0257-2. Epub 2010 Jan 21. Cell Mol Life Sci. 2010. PMID: 20091084 Free PMC article.
-
B-type nuclear lamin and the nuclear pore complex Nup107-160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis.Biol Open. 2016 Aug 15;5(8):1011-21. doi: 10.1242/bio.017566. Biol Open. 2016. PMID: 27402967 Free PMC article.
References
-
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. - PubMed
-
- Cao H., Hegele R.A. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 2000;9:109–112. - PubMed
-
- Collas I., Courvalin J.C. Sorting nuclear membrane proteins at mitosis. Trends Cell Biol. 2000;10:5–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

