An RGD sequence in the P2Y(2) receptor interacts with alpha(V)beta(3) integrins and is required for G(o)-mediated signal transduction
- PMID: 11331301
- PMCID: PMC2190579
- DOI: 10.1083/jcb.153.3.491
An RGD sequence in the P2Y(2) receptor interacts with alpha(V)beta(3) integrins and is required for G(o)-mediated signal transduction
Abstract
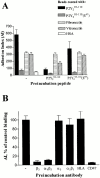
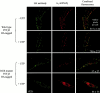
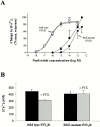
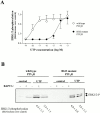
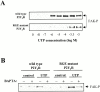
The P2Y(2) nucleotide receptor (P2Y(2)R) contains the integrin-binding domain arginine-glycine-aspartic acid (RGD) in its first extracellular loop, raising the possibility that this G protein-coupled receptor interacts directly with an integrin. Binding of a peptide corresponding to the first extracellular loop of the P2Y(2)R to K562 erythroleukemia cells was inhibited by antibodies against alpha(V)beta(3)/beta(5) integrins and the integrin-associated thrombospondin receptor, CD47. Immunofluorescence of cells transfected with epitope-tagged P2Y(2)Rs indicated that alpha(V) integrins colocalized 10-fold better with the wild-type P2Y(2)R than with a mutant P2Y(2)R in which the RGD sequence was replaced with RGE. Compared with the wild-type P2Y(2)R, the RGE mutant required 1,000-fold higher agonist concentrations to phosphorylate focal adhesion kinase, activate extracellular signal-regulated kinases, and initiate the PLC-dependent mobilization of intracellular Ca(2+). Furthermore, an anti-alpha(V) integrin antibody partially inhibited these signaling events mediated by the wild-type P2Y(2)R. Pertussis toxin, an inhibitor of G(i/o) proteins, partially inhibited Ca(2+) mobilization mediated by the wild-type P2Y(2)R, but not by the RGE mutant, suggesting that the RGD sequence is required for P2Y(2)R-mediated activation of G(o), but not G(q). Since CD47 has been shown to associate directly with G(i/o) family proteins, these results suggest that interactions between P2Y(2)Rs, integrins, and CD47 may be important for coupling the P2Y(2)R to G(o).
Figures
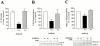

Similar articles
-
The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration.J Biol Chem. 2005 Nov 25;280(47):39050-7. doi: 10.1074/jbc.M504819200. Epub 2005 Sep 26. J Biol Chem. 2005. PMID: 16186116
-
The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12.J Cell Sci. 2007 May 1;120(Pt 9):1654-62. doi: 10.1242/jcs.03441. J Cell Sci. 2007. PMID: 17452627 Free PMC article.
-
Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors.J Biol Chem. 2004 Feb 27;279(9):8212-8. doi: 10.1074/jbc.M312230200. Epub 2003 Dec 11. J Biol Chem. 2004. PMID: 14670955
-
P2Y2 nucleotide receptor-mediated responses in brain cells.Mol Neurobiol. 2010 Jun;41(2-3):356-66. doi: 10.1007/s12035-010-8115-7. Epub 2010 Apr 13. Mol Neurobiol. 2010. PMID: 20387013 Free PMC article. Review.
-
Integrin-associated protein (CD47) and its ligands.Trends Cell Biol. 2001 Mar;11(3):130-5. doi: 10.1016/s0962-8924(00)01906-1. Trends Cell Biol. 2001. PMID: 11306274 Review.
Cited by
-
P2X7 receptors stimulate AKT phosphorylation in astrocytes.Br J Pharmacol. 2004 Apr;141(7):1106-17. doi: 10.1038/sj.bjp.0705685. Epub 2004 Mar 15. Br J Pharmacol. 2004. PMID: 15023862 Free PMC article.
-
P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells.J Biol Chem. 2010 Mar 5;285(10):7545-55. doi: 10.1074/jbc.M109.078170. Epub 2010 Jan 11. J Biol Chem. 2010. PMID: 20064929 Free PMC article.
-
Nucleotide signaling in nervous system development.Pflugers Arch. 2006 Aug;452(5):573-88. doi: 10.1007/s00424-006-0067-4. Epub 2006 Apr 25. Pflugers Arch. 2006. PMID: 16639549 Review.
-
P2 receptors: new potential players in atherosclerosis.Br J Pharmacol. 2002 Feb;135(4):831-42. doi: 10.1038/sj.bjp.0704524. Br J Pharmacol. 2002. PMID: 11861311 Free PMC article. Review.
-
CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model.Arthritis Res Ther. 2008;10(1):R4. doi: 10.1186/ar2350. Epub 2008 Jan 10. Arthritis Res Ther. 2008. PMID: 18186923 Free PMC article.
References
-
- Beekhuizen H., Van Furth R. Monocyte adherence to human vascular endothelium. J. Leukoc. Biol. 1993;54:363–378. - PubMed
-
- Boarder M.R., Weisman G.A., Turner J.T., Wilkinson G.F. G protein-coupled P2 purinoceptorsfrom molecular biology to functional responses. Trends Pharmacol. Sci. 1995;16:133–139. - PubMed
-
- Brooks P.C., Clark R.A., Cheresh D.A. Integrin αVβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels Cell. 79 1994. 1157 1165a - PubMed
-
- Brooks P.C., Clark R.A., Cheresh D.A. Requirement of vascular integrin αVβ3 for angiogenesis Science. 264 1994. 569 571b - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

