Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells
- PMID: 11331303
- PMCID: PMC2190575
- DOI: 10.1083/jcb.153.3.517
Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells
Abstract
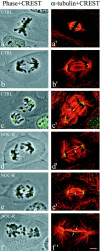
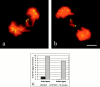
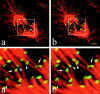
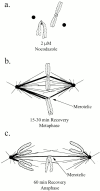
In mitotic cells, an error in chromosome segregation occurs when a chromosome is left near the spindle equator after anaphase onset (lagging chromosome). In PtK1 cells, we found 1.16% of untreated anaphase cells exhibiting lagging chromosomes at the spindle equator, and this percentage was enhanced to 17.55% after a mitotic block with 2 microM nocodazole. A lagging chromosome seen during anaphase in control or nocodazole-treated cells was found by confocal immunofluorescence microscopy to be a single chromatid with its kinetochore attached to kinetochore microtubule bundles extending toward opposite poles. This merotelic orientation was verified by electron microscopy. The single kinetochores of lagging chromosomes in anaphase were stretched laterally (1.2--5.6-fold) in the directions of their kinetochore microtubules, indicating that they were not able to achieve anaphase poleward movement because of pulling forces toward opposite poles. They also had inactivated mitotic spindle checkpoint activities since they did not label with either Mad2 or 3F3/2 antibodies. Thus, for mammalian cultured cells, kinetochore merotelic orientation is a major mechanism of aneuploidy not detected by the mitotic spindle checkpoint. The expanded and curved crescent morphology exhibited by kinetochores during nocodazole treatment may promote the high incidence of kinetochore merotelic orientation that occurs after nocodazole washout.
Figures




References
-
- Agard D.A., Hiraoka Y., Shaw P., Sedat J.W. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. - PubMed
-
- Canman J.C., Hoffman D., Salmon E.D. The role of pre- and post-anaphase microtubules in the cytokinetic phase, or C-phase, of the cell cycle. Curr. Biol. 2000;10:611–614. - PubMed
-
- Cimini D., Antoccia A., Tanzarella C., Degrassi F. Topoisomerase II inhibition in mitosis produces numerical and structural chromosomal aberrations in human fibroblasts. Cytogenet. Cell Genet. 1997;76:61–67. - PubMed
-
- Cimini D., Tanzarella C., Degrassi F. Differences in malsegregation rates obtained by scoring ana-telophase or binucleate cells. Mutagenesis. 1999;14:563–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

