Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland
- PMID: 11331306
- PMCID: PMC2190562
- DOI: 10.1083/jcb.153.3.555
Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland
Abstract
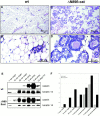
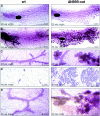
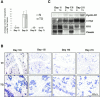
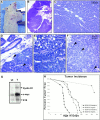
To investigate the role of beta-catenin in mammary gland development and neoplasia, we expressed a stabilized, transcriptionally active form of beta-catenin lacking the NH(2)-terminal 89 amino acids (Delta N 89 beta-catenin) under the control of the mouse mammary tumor virus long terminal repeat. Our results show that Delta N 89 beta-catenin induces precocious lobuloalveolar development and differentiation in the mammary glands of both male and female mice. Virgin Delta N 89 beta-catenin mammary glands resemble those found in wild-type (wt) pregnant mice and inappropriately express cyclin D1 mRNA. In contrast to wt mammary glands, which resume a virgin appearance after cessation of lactation, transgenic mammary glands involute to a midpregnant status. All transgenic females develop multiple aggressive adenocarcinomas early in life. Surprisingly, the Delta N89 beta-catenin phenotype differs from those elicited by overexpression of Wnt genes in this gland. In particular, Delta N 89 beta-catenin has no effect on ductal side branching. This suggests that Wnt induction of ductal branching involves additional downstream effectors or modulators.
Figures





Similar articles
-
Constitutive overexpression of Id-1 in mammary glands of transgenic mice results in precocious and increased formation of terminal end buds, enhanced alveologenesis, delayed involution.J Cell Physiol. 2011 May;226(5):1340-52. doi: 10.1002/jcp.22462. J Cell Physiol. 2011. PMID: 20945346
-
beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland.Oncogene. 2001 Aug 23;20(37):5093-9. doi: 10.1038/sj.onc.1204586. Oncogene. 2001. PMID: 11526497
-
Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia.Development. 2005 Jan;132(2):267-77. doi: 10.1242/dev.01583. Epub 2004 Dec 8. Development. 2005. PMID: 15590737
-
Beta-catenin and Tcfs in mammary development and cancer.J Mammary Gland Biol Neoplasia. 2003 Apr;8(2):145-58. doi: 10.1023/a:1025944723047. J Mammary Gland Biol Neoplasia. 2003. PMID: 14635791 Review.
-
[Mammary gland development: Role of basal myoepithelial cells].J Soc Biol. 2006;200(2):193-8. doi: 10.1051/jbio:2006021. J Soc Biol. 2006. PMID: 17151555 Review. French.
Cited by
-
R-spondin-3 is an oncogenic driver of poorly differentiated invasive breast cancer.J Pathol. 2022 Nov;258(3):289-299. doi: 10.1002/path.5999. Epub 2022 Sep 15. J Pathol. 2022. PMID: 36106661 Free PMC article.
-
Wnt signaling in mammary glands: plastic cell fates and combinatorial signaling.Cold Spring Harb Perspect Biol. 2012 Oct 1;4(10):a008037. doi: 10.1101/cshperspect.a008037. Cold Spring Harb Perspect Biol. 2012. PMID: 22661590 Free PMC article. Review.
-
Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells.Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15853-8. doi: 10.1073/pnas.2136825100. Epub 2003 Dec 10. Proc Natl Acad Sci U S A. 2003. PMID: 14668450 Free PMC article.
-
The transcription factor ATF3 acts as an oncogene in mouse mammary tumorigenesis.BMC Cancer. 2008 Sep 22;8:268. doi: 10.1186/1471-2407-8-268. BMC Cancer. 2008. PMID: 18808719 Free PMC article.
-
STAT3 upregulates the protein expression and transcriptional activity of β-catenin in breast cancer.Int J Clin Exp Pathol. 2010 Jul 25;3(7):654-64. Int J Clin Exp Pathol. 2010. PMID: 20830236 Free PMC article.
References
-
- Behrens J., von Kries J.P., Kuhl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
-
- Buckley A., Middleton M.C. Retinoic acid alters the keratinization of cultured rat sublingual keratinocytes in vitro. Arch. Dermatol. Res. 1987;279:257–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

