Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways
- PMID: 11331310
- PMCID: PMC2190566
- DOI: 10.1083/jcb.153.3.613
Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA damage-signaling pathways
Erratum in
- J Cell Biol 2001 Jul 23;154(2):469
Abstract
The tumor suppressor p53 binding protein 1 (53BP1) binds to the DNA-binding domain of p53 and enhances p53-mediated transcriptional activation. 53BP1 contains two breast cancer susceptibility gene 1 COOH terminus (BRCT) motifs, which are present in several proteins involved in DNA repair and/or DNA damage-signaling pathways. Thus, we investigated the potential role of 53BP1 in DNA damage-signaling pathways. Here, we report that 53BP1 becomes hyperphosphorylated and forms discrete nuclear foci in response to DNA damage. These foci colocalize at all time points with phosphorylated H2AX (gamma-H2AX), which has been previously demonstrated to localize at sites of DNA strand breaks. 53BP1 foci formation is not restricted to gamma-radiation but is also detected in response to UV radiation as well as hydroxyurea, camptothecin, etoposide, and methylmethanesulfonate treatment. Several observations suggest that 53BP1 is regulated by ataxia telangiectasia mutated (ATM) after DNA damage. First, ATM-deficient cells show no 53BP1 hyperphosphorylation and reduced 53BP1 foci formation in response to gamma-radiation compared with cells expressing wild-type ATM. Second, wortmannin treatment strongly inhibits gamma-radiation-induced hyperphosphorylation and foci formation of 53BP1. Third, 53BP1 is readily phosphorylated by ATM in vitro. Taken together, these results suggest that 53BP1 is an ATM substrate that is involved early in the DNA damage-signaling pathways in mammalian cells.
Figures


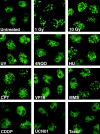
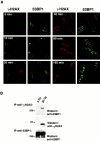
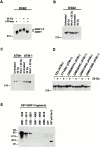
Similar articles
-
Phosphorylation and rapid relocalization of 53BP1 to nuclear foci upon DNA damage.Mol Cell Biol. 2001 Mar;21(5):1719-29. doi: 10.1128/MCB.21.5.1719-1729.2001. Mol Cell Biol. 2001. PMID: 11238909 Free PMC article.
-
p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks.J Cell Biol. 2000 Dec 25;151(7):1381-90. doi: 10.1083/jcb.151.7.1381. J Cell Biol. 2000. PMID: 11134068 Free PMC article.
-
53BP1 and NFBD1/MDC1-Nbs1 function in parallel interacting pathways activating ataxia-telangiectasia mutated (ATM) in response to DNA damage.Cancer Res. 2003 Dec 15;63(24):8586-91. Cancer Res. 2003. PMID: 14695167
-
ATM signaling and 53BP1.Radiother Oncol. 2005 Aug;76(2):119-22. doi: 10.1016/j.radonc.2005.06.026. Radiother Oncol. 2005. PMID: 16024119 Review.
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
Cited by
-
XRN2 Links Transcription Termination to DNA Damage and Replication Stress.PLoS Genet. 2016 Jul 20;12(7):e1006107. doi: 10.1371/journal.pgen.1006107. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27437695 Free PMC article.
-
Luminal progenitor and mature cells are more susceptible than basal cells to radiation-induced DNA double-strand breaks in rat mammary tissue.J Radiat Res. 2024 Sep 24;65(5):640-650. doi: 10.1093/jrr/rrae067. J Radiat Res. 2024. PMID: 39238338 Free PMC article.
-
Activation of the ATR pathway by human immunodeficiency virus type 1 Vpr involves its direct binding to chromatin in vivo.J Virol. 2005 Dec;79(24):15443-51. doi: 10.1128/JVI.79.24.15443-15451.2005. J Virol. 2005. PMID: 16306615 Free PMC article.
-
ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells.Mol Biol Cell. 2005 Oct;16(10):5013-25. doi: 10.1091/mbc.e05-01-0065. Epub 2005 Jul 19. Mol Biol Cell. 2005. PMID: 16030261 Free PMC article.
-
Structure of the 53BP1 BRCT region bound to p53 and its comparison to the Brca1 BRCT structure.Genes Dev. 2002 Mar 1;16(5):583-93. doi: 10.1101/gad.959202. Genes Dev. 2002. PMID: 11877378 Free PMC article.
References
-
- Appella E., Anderson C.W. Signaling to p53breaking the posttranslational modification code. Pathol. Biol. (Paris) 2000;48:227–245. - PubMed
-
- Bork P., Hofmann K., Bucher P., Neuwald A.F., Altschul S.F., Koonin E.V. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. - PubMed
-
- Callebaut I., Mornon J.P. From BRCA1 to RAP1a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. - PubMed
-
- Canman C.E., Lim D.S., Cimprich K.A., Taya Y., Tamai K., Sakaguchi K., Appella E., Kastan M.B., Siliciano J.D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. - PubMed
-
- Chai Y.L., Cui J., Shao N., Shyam E., Reddy P., Rao V.N. The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter. Oncogene. 1999;18:263–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

