ATP regulation of recombinant type 3 inositol 1,4,5-trisphosphate receptor gating
- PMID: 11331355
- PMCID: PMC2233659
- DOI: 10.1085/jgp.117.5.447
ATP regulation of recombinant type 3 inositol 1,4,5-trisphosphate receptor gating
Abstract
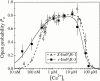
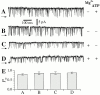
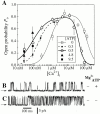
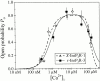
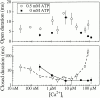
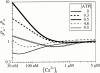
A family of inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) Ca2+ release channels plays a central role in Ca2+ signaling in most cells, but functional correlates of isoform diversity are unclear. Patch-clamp electrophysiology of endogenous type 1 (X-InsP3R-1) and recombinant rat type 3 InsP3R (r-InsP3R-3) channels in the outer membrane of isolated Xenopus oocyte nuclei indicated that enhanced affinity and reduced cooperativity of Ca2+ activation sites of the InsP3-liganded type 3 channel distinguished the two isoforms. Because Ca2+ activation of type 1 channel was the target of regulation by cytoplasmic ATP free acid concentration ([ATP](i)), here we studied the effects of [ATP]i on the dependence of r-InsP(3)R-3 gating on cytoplasmic free Ca2+ concentration ([Ca2+]i. As [ATP]i was increased from 0 to 0.5 mM, maximum r-InsP3R-3 channel open probability (Po) remained unchanged, whereas the half-maximal activating [Ca2+]i and activation Hill coefficient both decreased continuously, from 800 to 77 nM and from 1.6 to 1, respectively, and the half-maximal inhibitory [Ca2+]i was reduced from 115 to 39 microM. These effects were largely due to effects of ATP on the mean closed channel duration. Whereas the r-InsP3R-3 had a substantially higher Po than X-InsP3R-1 in activating [Ca2+]i (< 1 microM) and 0.5 mM ATP, the Ca2+ dependencies of channel gating of the two isoforms became remarkably similar in the absence of ATP. Our results suggest that ATP binding is responsible for conferring distinct gating properties on the two InsP3R channel isoforms. Possible molecular models to account for the distinct regulation by ATP of the Ca2+ activation properties of the two channel isoforms and the physiological implications of these results are discussed. Complex regulation by ATP of the types 1 and 3 InsP3R channel activities may enable cells to generate sophisticated patterns of Ca2+ signals with cytoplasmic ATP as one of the second messengers.
Figures

Comment in
-
Subtype-specific regulation of inositol 1,4,5-trisphosphate receptors: controlling calcium signals in time and space.J Gen Physiol. 2001 May;117(5):431-4. doi: 10.1085/jgp.117.5.431. J Gen Physiol. 2001. PMID: 11331353 Free PMC article. Review. No abstract available.
Similar articles
-
Spontaneous channel activity of the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R). Application of allosteric modeling to calcium and InsP3 regulation of InsP3R single-channel gating.J Gen Physiol. 2003 Nov;122(5):583-603. doi: 10.1085/jgp.200308809. J Gen Physiol. 2003. PMID: 14581584 Free PMC article.
-
Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 insp3 receptors.J Gen Physiol. 2001 May;117(5):435-46. doi: 10.1085/jgp.117.5.435. J Gen Physiol. 2001. PMID: 11331354 Free PMC article.
-
ATP-dependent adenophostin activation of inositol 1,4,5-trisphosphate receptor channel gating: kinetic implications for the durations of calcium puffs in cells.J Gen Physiol. 2001 Apr;117(4):299-314. doi: 10.1085/jgp.117.4.299. J Gen Physiol. 2001. PMID: 11279251 Free PMC article.
-
The inositol trisphosphate receptor of Xenopus oocytes.Cell Calcium. 1995 Nov;18(5):353-63. doi: 10.1016/0143-4160(95)90051-9. Cell Calcium. 1995. PMID: 8581964 Review.
-
[Molecular and functional diversity of inositol triphosphate-induced Ca(2+) release].Verh K Acad Geneeskd Belg. 1995;57(5):423-58. Verh K Acad Geneeskd Belg. 1995. PMID: 8571671 Review. Dutch.
Cited by
-
Spontaneous channel activity of the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R). Application of allosteric modeling to calcium and InsP3 regulation of InsP3R single-channel gating.J Gen Physiol. 2003 Nov;122(5):583-603. doi: 10.1085/jgp.200308809. J Gen Physiol. 2003. PMID: 14581584 Free PMC article.
-
ATP regulation of type-1 inositol 1,4,5-trisphosphate receptor activity does not require walker A-type ATP-binding motifs.J Biol Chem. 2009 Jun 12;284(24):16156-16163. doi: 10.1074/jbc.M109.006452. Epub 2009 Apr 22. J Biol Chem. 2009. PMID: 19386591 Free PMC article.
-
Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region.Biophys J. 2005 Feb;88(2):1056-69. doi: 10.1529/biophysj.104.049601. Epub 2004 Nov 5. Biophys J. 2005. PMID: 15531634 Free PMC article.
-
Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases.Prog Retin Eye Res. 2009 May;28(3):155-77. doi: 10.1016/j.preteyeres.2009.04.003. Epub 2009 Apr 17. Prog Retin Eye Res. 2009. PMID: 19376264 Free PMC article. Review.
-
From IP3RPEP6 Inhibition of IP3 receptor channels to insights: do channel subunits collaborate or cooperate?Cell Mol Life Sci. 2025 Jul 19;82(1):285. doi: 10.1007/s00018-025-05813-7. Cell Mol Life Sci. 2025. PMID: 40682657 Free PMC article. Review.
References
-
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Bezprozvanny I., Ehrlich B.E. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron. 1993;10:1175–1184. - PubMed
-
- Bezprozvanny I., Ehrlich B.E. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 1995;145:205–216. - PubMed
-
- Changeux J.-P., Edelstein S.J. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. - PubMed
-
- Corkey B.E., Duszynski J., Rich T.L., Matschinsky B., Williamson J.R. Regulation of free and bound magnesium in rat hepatocytes and isolated mitochondria. J. Biol. Chem. 1986;261:2567–2574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

