Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity
- PMID: 11331361
- PMCID: PMC6762471
- DOI: 10.1523/JNEUROSCI.21-10-03322.2001
Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity
Abstract
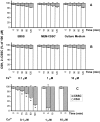
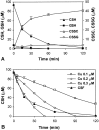
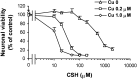
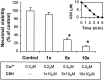
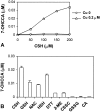
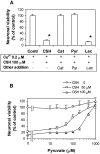
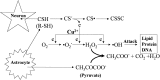
We have found previously that astrocytes can provide cysteine to neurons. However, cysteine has been reported to be neurotoxic although it plays a pivotal role in regulating intracellular levels of glutathione, the major cellular antioxidant. Here, we show that cysteine toxicity is a result of hydroxyl radicals generated during cysteine autoxidation. Transition metal ions are candidates to catalyze this process. Copper substantially accelerates the autoxidation rate of cysteine even at submicromolar levels, whereas iron and other transition metal ions, including manganese, chromium, and zinc, are less efficient. The autoxidation rate of cysteine in rat CSF is equal to that observed in the presence of approximately 0.2 microm copper. In tissue culture tests, we found that cysteine toxicity depends highly on its autoxidation rate and on the total amount of cysteine being oxidized, suggesting that the toxicity can be attributed to the free radicals produced from cysteine autoxidation, but not to cysteine itself. We have also explored the in vivo mechanisms that protect against cysteine toxicity. Catalase and pyruvate were each found to inhibit the production of hydroxyl radicals generated by cysteine autoxidation. In tissue culture, they both protected primary neurons against cysteine toxicity catalyzed by copper. This protection is attributed to their ability to react with hydrogen peroxide, preventing the formation of hydroxyl radicals. Pyruvate, but not catalase or glutathione peroxidase, was detected in astrocyte-conditioned medium and CSF. Our data therefore suggest that astrocytes can prevent cysteine toxicity by releasing pyruvate.
Figures



Similar articles
-
Astrocytes provide cysteine to neurons by releasing glutathione.J Neurochem. 2000 Apr;74(4):1434-42. doi: 10.1046/j.1471-4159.2000.0741434.x. J Neurochem. 2000. PMID: 10737599
-
Protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism.Mol Biol Rep. 2011 Jun;38(5):3235-42. doi: 10.1007/s11033-010-9998-0. Epub 2010 Feb 24. Mol Biol Rep. 2011. PMID: 20182801
-
Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity.J Neurosci Res. 2006 Aug 15;84(3):578-86. doi: 10.1002/jnr.20957. J Neurosci Res. 2006. PMID: 16721761
-
Metals, toxicity and oxidative stress.Curr Med Chem. 2005;12(10):1161-208. doi: 10.2174/0929867053764635. Curr Med Chem. 2005. PMID: 15892631 Review.
-
Transition metals as catalysts of "autoxidation" reactions.Free Radic Biol Med. 1990;8(1):95-108. doi: 10.1016/0891-5849(90)90148-c. Free Radic Biol Med. 1990. PMID: 2182396 Review.
Cited by
-
Modulation of Pyruvate Export and Extracellular Pyruvate Concentration in Primary Astrocyte Cultures.Neurochem Res. 2024 May;49(5):1331-1346. doi: 10.1007/s11064-024-04120-0. Epub 2024 Feb 20. Neurochem Res. 2024. PMID: 38376749 Free PMC article.
-
A seminaphthofluorescein-based fluorescent chemodosimeter for the highly selective detection of cysteine.Org Biomol Chem. 2012 Apr 14;10(14):2739-41. doi: 10.1039/c2ob25178g. Epub 2012 Mar 5. Org Biomol Chem. 2012. PMID: 22391857 Free PMC article.
-
Nanotechnology-mediated crossing of two impermeable membranes to modulate the stars of the neurovascular unit for neuroprotection.Proc Natl Acad Sci U S A. 2018 Dec 26;115(52):E12333-E12342. doi: 10.1073/pnas.1816429115. Epub 2018 Dec 10. Proc Natl Acad Sci U S A. 2018. PMID: 30530697 Free PMC article.
-
Cu/Zn-superoxide dismutase and wild-type like fALS SOD1 mutants produce cytotoxic quantities of H2O2 via cysteine-dependent redox short-circuit.Sci Rep. 2019 Jul 25;9(1):10826. doi: 10.1038/s41598-019-47326-x. Sci Rep. 2019. PMID: 31346243 Free PMC article.
-
Structure and function of CinD (YtjD) of Lactococcus lactis, a copper-induced nitroreductase involved in defense against oxidative stress.J Bacteriol. 2010 Aug;192(16):4172-80. doi: 10.1128/JB.00372-10. Epub 2010 Jun 18. J Bacteriol. 2010. PMID: 20562311 Free PMC article.
References
-
- Aguilar MV, Jimenez-Jimenez FJ, Molina JA, Meseguer I, Mateos-Vega CJ, Gonzalez-Munoz MJ, de Bustos F, Gomez-Escalonilla C, Ort-Pareja M, Zurdo M, Martinez-Para MC. Cerebrospinal fluid selenium and chromium levels in patients with Parkinson's disease. J Neural Transm. 1998;105:1245–1251. - PubMed
-
- Beutler E. Nutritional and metabolic aspects of glutathione. Annu Rev Nutr. 1989;9:287–302. - PubMed
-
- Bunton CA. Oxidation of α-diketones and α-keto-acids by hydrogen peroxide. Nature. 1949;163:444. - PubMed
-
- Cavallini D, De Marco C, Dupre S, Rotilio G. The copper catalyzed oxidation of cysteine to cystine. Arch Biochem Biophys. 1969;130:354–361. - PubMed
-
- Chevion M. A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic Biol Med. 1988;5:27–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
