Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2
- PMID: 11331365
- PMCID: PMC6762489
- DOI: 10.1523/JNEUROSCI.21-10-03360.2001
Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2
Abstract
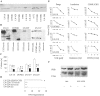
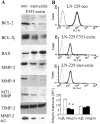
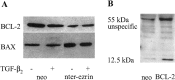
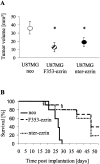
Ezrin belongs to the ezrin-radixin-moesin family proteins, which cross-link actin cytoskeleton and plasma membrane. Malignant glioma cells are paradigmatic for their strong migratory and invasive properties. Here, we report that the expression of dominant-negative ezrins inhibits clonogenicity, migration, and invasiveness of human malignant glioma cells. Furthermore, dominant-negative ezrins block hepatocyte growth factor (HGF)-mediated stimulation of clonogenicity and migration, without altering HGF-induced protein kinase B/Akt and focal adhesion kinase phosphorylation. Glioma cells expressing dominant-negative ezrins exhibit a shift of the BCL-2/BAX rheostat toward apoptosis, reduced alpha(V)beta(3) integrin expression and reduced matrix metalloproteinase (MMP) expression and activity. These changes are associated with a dramatic loss of transforming growth factor beta(2) (TGF-beta(2)) release. Exogenous supplementation of TGF-beta(2) overcomes the inhibitory effects of dominant-negative ezrins on migration and clonogenicity. A neutralizing TGF-beta(2) antibody mimics the effects of dominant-negative ezrins on clonogenicity and migration. Exogenous HGF markedly induces TGF-beta(2) protein levels, and a neutralizing TGF-beta(2) antibody abolishes the HGF-mediated increase in glioma cell motility. Finally, TGF-beta(2) does not modulate BCL-2 or BAX expression, but BCL-2 gene transfer increases the levels of latent and active TGF-beta(2). Intracranial xenografts of U87MG glioma cells transfected with the dominant-negative ezrins in athymic mice grow to significantly smaller volumes, and the median survival of these mice is 50 d compared with 28 d in the control group. These data define a novel pathway for HGF-induced glioma cell migration and invasion, which requires ezrin, changes in the BCL-2/BAX rheostat, and the induction of TGF-beta(2) expression in vitro, and underscore the important role of HGF signaling in vivo.
Figures


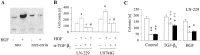
Similar articles
-
Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta.J Neurooncol. 2001 Jun;53(2):177-85. doi: 10.1023/a:1012209518843. J Neurooncol. 2001. PMID: 11716069 Review.
-
BCL-2-induced glioma cell invasiveness depends on furin-like proteases.J Neurochem. 2004 Dec;91(6):1275-83. doi: 10.1111/j.1471-4159.2004.02806.x. J Neurochem. 2004. PMID: 15584904
-
BCL-xL: time-dependent dissociation between modulation of apoptosis and invasiveness in human malignant glioma cells.Cell Death Differ. 2006 Jul;13(7):1156-69. doi: 10.1038/sj.cdd.4401786. Epub 2005 Oct 28. Cell Death Differ. 2006. PMID: 16254573
-
SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo.Cancer Res. 2004 Nov 1;64(21):7954-61. doi: 10.1158/0008-5472.CAN-04-1013. Cancer Res. 2004. PMID: 15520202
-
Recent Advances in Understanding Mechanisms of TGF Beta Signaling and Its Role in Glioma Pathogenesis.Adv Exp Med Biol. 2020;1202:179-201. doi: 10.1007/978-3-030-30651-9_9. Adv Exp Med Biol. 2020. PMID: 32034714 Review.
Cited by
-
Rosiglitazone suppresses glioma cell growth and cell cycle by blocking the transforming growth factor-beta mediated pathway.Neurochem Res. 2012 Oct;37(10):2076-84. doi: 10.1007/s11064-012-0828-8. Epub 2012 Jun 16. Neurochem Res. 2012. PMID: 22707243
-
Reciprocal regulation by estradiol 17-beta of ezrin and cadherin-catenin complexes in pituitary GH3 cells.Endocrine. 2002 Apr;17(3):219-28. doi: 10.1385/ENDO:17:3:219. Endocrine. 2002. PMID: 12108523
-
Huntingtin-Interacting Protein 1-Related (HIP1R) Regulates Rheumatoid Arthritis Synovial Fibroblast Invasiveness.Cells. 2025 Mar 23;14(7):483. doi: 10.3390/cells14070483. Cells. 2025. PMID: 40214437 Free PMC article.
-
Mechanisms underlying cancer progression caused by ezrin overexpression in tongue squamous cell carcinoma.PLoS One. 2013;8(1):e54881. doi: 10.1371/journal.pone.0054881. Epub 2013 Jan 24. PLoS One. 2013. PMID: 23357878 Free PMC article.
-
The expression of ezrin in NPC and its interaction with NGX6, a novel candidate suppressor.Cancer Sci. 2007 Mar;98(3):341-9. doi: 10.1111/j.1349-7006.2007.00410.x. Cancer Sci. 2007. PMID: 17270023 Free PMC article.
References
-
- Beviglia L, Kramer R. HGF induces FAK activation and integrin-mediated adhesion in MTLn3 breast carcinoma cells. Int J Cancer. 1999;83:640–649. - PubMed
-
- Bourguignon LY, Zhu H, Shao L, Chen YW (2000) CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid (HA)-dependent ovarian tumor cell migration. J Biol Chem: Nov 17, epub ahead of print. - PubMed
-
- Brooks P, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αVβ3. Cell. 1996;85:683–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
