Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice
- PMID: 11331371
- PMCID: PMC6762474
- DOI: 10.1523/JNEUROSCI.21-10-03409.2001
Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice
Abstract
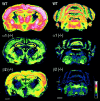
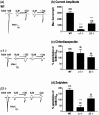
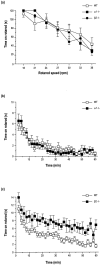
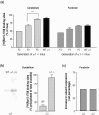
The alpha1beta2gamma2 is the most abundant subtype of the GABA(A) receptor and is localized in many regions of the brain. To gain more insight into the role of this receptor subtype in the modulation of inhibitory neurotransmission, we generated mice lacking either the alpha1 or beta2 subunit. In agreement with the reported abundance of this subtype, >50% of total GABA(A) receptors are lost in both alpha1-/- and beta2-/- mice. Surprisingly, homozygotes of both mouse lines are viable, fertile, and show no spontaneous seizures. Initially half of the alpha1-/- mice died prenatally or perinatally, but they exhibited a lower mortality rate in subsequent generations, suggesting some phenotypic drift and adaptive changes. Both adult alpha1-/- and beta2-/- mice demonstrate normal performances on the rotarod, but beta2-/- mice displayed increased locomotor activity. Purkinje cells of the cerebellum primarily express alpha1beta2gamma2 receptors, and in electrophysiological recordings from alpha1-/- mice GABA currents in these neurons are dramatically reduced, and residual currents have a benzodiazepine pharmacology characteristic of alpha2- or alpha3-containing receptors. In contrast, the cerebellar Purkinje neurons from beta2-/- mice have only a relatively small reduction of GABA currents. In beta2-/- mice expression levels of all six alpha subunits are reduced by approximately 50%, suggesting that the beta2 subunit can coassemble with alpha subunits other than just alpha1. Our data confirm that alpha1beta2gamma2 is the major GABA(A) receptor subtype in the murine brain and demonstrate that, surprisingly, the loss of this receptor subtype is not lethal.
Figures


References
-
- Baer K, Essrich C, Balsiger S, Wick MJ, Harris RA, Fritschy J-M, Luscher B. Rescue of γ2 subunit-deficient mice by transgenic overexpression of the GABAA receptor γ2S or γ2L subunit isoforms. Eur J Neurosci. 2000;12:2639–2643. - PubMed
-
- Barnard EA, Skolnick P, Olson RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
-
- Benke D, Fritschy J-M, Trzeciak A, Bannwarth W, Mohler H. Distribution, prevalence, and drug binding profile of γ-aminobutyric acid type A receptor subtypes differing in the β-subunit variant. J Biol Chem. 1994;269:27100–27107. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent K+ conductance. Nature. 2001;409:88–92. - PubMed
-
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy J-M, Luscher B, Mohler H. Decreased GABAA receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
