Suppression of neuronal hyperexcitability and associated delayed neuronal death by adenoviral expression of GABA(C) receptors
- PMID: 11331372
- PMCID: PMC6762476
- DOI: 10.1523/JNEUROSCI.21-10-03419.2001
Suppression of neuronal hyperexcitability and associated delayed neuronal death by adenoviral expression of GABA(C) receptors
Abstract
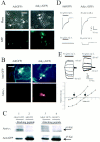
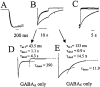
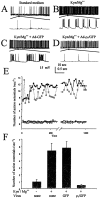
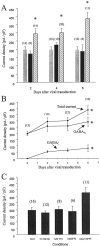
The excessive neuronal excitation underlying several clinically important diseases is often treated with GABA allosteric modulators in an attempt to enhance inhibition. An alternative strategy would be to enhance directly the sensitivity of postsynaptic neurons to GABA. The GABA(C) receptor, normally found only in the retina, is more sensitive to GABA and demonstrates little desensitization compared with the GABA(A) receptor. We constructed an adenovirus vector that expressed cDNA for both the GABA(C) receptor rho(1) subunit and a green fluorescent protein (GFP) reporter and used it to transduce cultured hippocampal neurons. Transduced neurons were identified by fluorescence, double immunocytochemistry proved colocalization of the rho(1) protein and the reporter, Western blot verified the expected molecular masses, and electrophysiological and pharmacological properties confirmed the presence of functional GABA(C) receptors. rho(1)-GFP transduction resulted in an increased density of GABA(A) receptors as well as expression of novel GABA(C) receptors. This effect was not reproduced by addition of TTX or Mg(2+) to the culture medium to reduce action potentials or synaptic activity. In a model of neuronal hyperexcitability induced by chronic blockade of glutamate receptors, expression of GABA(C) receptors abolished the hyperactivity and the consequent delayed neuronal death. Adenovirus-mediated neuronal GABA(C) receptor engineering, via its dual mechanism of inhibition, may offer a way of inhibiting only those hyperexcitable neurons responsible for clinical problems, avoiding the generalized nervous system depression associated with pharmacological therapy.
Figures


Similar articles
-
Recombinant GABA(C) receptors expressed in rat hippocampal neurons after infection with an adenovirus containing the human rho1 subunit.J Physiol. 2001 Aug 15;535(Pt 1):145-53. doi: 10.1111/j.1469-7793.2001.00145.x. J Physiol. 2001. PMID: 11507165 Free PMC article.
-
GABA(C) rho(1) subunits form functional receptors but not functional synapses in hippocampal neurons.J Neurophysiol. 2001 Nov;86(5):2605-15. doi: 10.1152/jn.2001.86.5.2605. J Neurophysiol. 2001. PMID: 11698546
-
Synaptically-silent immature neurons show gaba and glutamate receptor-mediated currents in adult rat dentate gyrus.Arch Ital Biol. 2006 May;144(2):115-26. Arch Ital Biol. 2006. PMID: 16642790
-
Glutamate hyperexcitability and seizure-like activity throughout the brain and spinal cord upon relief from chronic glutamate receptor blockade in culture.Neuroscience. 1996 Oct;74(3):653-74. doi: 10.1016/0306-4522(96)00153-4. Neuroscience. 1996. PMID: 8884763
-
GABA(A) and GABA(B) receptors have opposite effects on synaptic glutamate release on the nucleus tractus solitarii neurons.Neuroscience. 2012 May 3;209:39-46. doi: 10.1016/j.neuroscience.2012.02.025. Epub 2012 Feb 22. Neuroscience. 2012. PMID: 22410341
Cited by
-
Kinetic properties of GABA rho1 homomeric receptors expressed in HEK293 cells.Biophys J. 2006 Sep 15;91(6):2155-62. doi: 10.1529/biophysj.106.085431. Epub 2006 Jun 23. Biophys J. 2006. PMID: 16798806 Free PMC article.
-
Recombinant GABA(C) receptors expressed in rat hippocampal neurons after infection with an adenovirus containing the human rho1 subunit.J Physiol. 2001 Aug 15;535(Pt 1):145-53. doi: 10.1111/j.1469-7793.2001.00145.x. J Physiol. 2001. PMID: 11507165 Free PMC article.
-
Chemogenetics of cell surface receptors: beyond genetic and pharmacological approaches.RSC Chem Biol. 2022 Jan 27;3(3):269-287. doi: 10.1039/d1cb00195g. eCollection 2022 Mar 9. RSC Chem Biol. 2022. PMID: 35359495 Free PMC article. Review.
-
Molecular Tools for Targeted Control of Nerve Cell Electrical Activity. Part II.Acta Naturae. 2021 Oct-Dec;13(4):17-32. doi: 10.32607/actanaturae.11415. Acta Naturae. 2021. PMID: 35127143 Free PMC article.
-
Chemogenetic Tools for Causal Cellular and Neuronal Biology.Physiol Rev. 2018 Jan 1;98(1):391-418. doi: 10.1152/physrev.00009.2017. Physiol Rev. 2018. PMID: 29351511 Free PMC article. Review.
References
-
- Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;2:413–419. - PubMed
-
- Amin J, Weiss DS. Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. - PubMed
-
- Amin J, Weiss DS. Insights into the activation mechanism of ρ1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits. Proc R Soc Lond B Biol Sci. 1996;263:273–282. - PubMed
-
- Bormann J. The “ABC” of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
