Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid
- PMID: 11331375
- PMCID: PMC6762497
- DOI: 10.1523/JNEUROSCI.21-10-03457.2001
Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid
Abstract
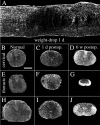
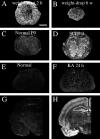
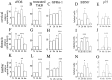
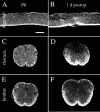
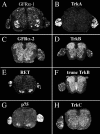
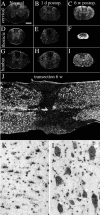
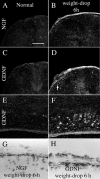
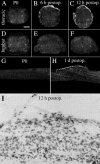
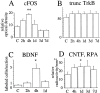
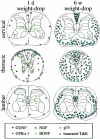
Delivery of neurotrophic factors to the injured spinal cord has been shown to stimulate neuronal survival and regeneration. This indicates that a lack of sufficient trophic support is one factor contributing to the absence of spontaneous regeneration in the mammalian spinal cord. Regulation of the expression of neurotrophic factors and receptors after spinal cord injury has not been studied in detail. We investigated levels of mRNA-encoding neurotrophins, glial cell line-derived neurotrophic factor (GDNF) family members and related receptors, ciliary neurotrophic factor (CNTF), and c-fos in normal and injured spinal cord. Injuries in adult rats included weight-drop, transection, and excitotoxic kainic acid delivery; in newborn rats, partial transection was performed. The regulation of expression patterns in the adult spinal cord was compared with that in the PNS and the neonate spinal cord. After mechanical injury of the adult rat spinal cord, upregulations of NGF and GDNF mRNA occurred in meningeal cells adjacent to the lesion. BDNF and p75 mRNA increased in neurons, GDNF mRNA increased in astrocytes close to the lesion, and GFRalpha-1 and truncated TrkB mRNA increased in astrocytes of degenerating white matter. The relatively limited upregulation of neurotrophic factors in the spinal cord contrasted with the response of affected nerve roots, in which marked increases of NGF and GDNF mRNA levels were observed in Schwann cells. The difference between the ability of the PNS and CNS to provide trophic support correlates with their different abilities to regenerate. Kainic acid delivery led to only weak upregulations of BDNF and CNTF mRNA. Compared with several brain regions, the overall response of the spinal cord tissue to kainic acid was weak. The relative sparseness of upregulations of endogenous neurotrophic factors after injury strengthens the hypothesis that lack of regeneration in the spinal cord is attributable at least partly to lack of trophic support.
Figures







References
-
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. - PubMed
-
- Baloh R, Tansey M, Golden J, Creedon D, Heuckeroth R, Keck C, Zimonjic D, Popescu N, Johnson E, Jr, Milbrandt J. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18:793–802. - PubMed
-
- Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors: implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
