Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor
- PMID: 11331377
- PMCID: PMC6762483
- DOI: 10.1523/JNEUROSCI.21-10-03483.2001
Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor
Abstract
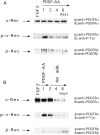
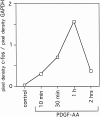
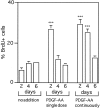
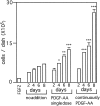
Identifying external signals involved in the regulation of neural stem cell proliferation and differentiation is fundamental to the understanding of CNS development. In this study we show that platelet-derived growth factor (PDGF) can act as a mitogen for neural precursor cells. Multipotent stem cells from developing CNS can be maintained in a proliferative state under serum-free conditions in the presence of fibroblast growth factor-2 (FGF2) and induced to differentiate into neurons, astrocytes, and oligodendrocytes on withdrawal of the mitogen. PDGF has been suggested to play a role during the differentiation into neurons. We have investigated the effect of PDGF on cultured stem cells from embryonic rat cortex. The PDGF alpha-receptor is constantly expressed during differentiation of neural stem cells but is phosphorylated only after PDGF-AA treatment. In contrast, the PDGF beta-receptor is hardly detectable in uncommitted cells, but its expression increases during differentiation. We show that PDGF stimulation leads to c-fos induction, 5'-bromo-2'deoxyuridine incorporation, and an increase in the number of immature cells stained with antibodies to neuronal markers. Our findings suggest that PDGF acts as a mitogen in the early phase of stem cell differentiation to expand the pool of immature neurons.
Figures



Similar articles
-
Autocrine/paracrine platelet-derived growth factor regulates proliferation of neural progenitor cells.Cancer Res. 2006 Aug 15;66(16):8042-8. doi: 10.1158/0008-5472.CAN-06-0900. Cancer Res. 2006. PMID: 16912180
-
Extracellular signal-regulated protein kinase signaling is uncoupled from initial differentiation of central nervous system stem cells to neurons.Mol Cancer Res. 2002 Dec;1(2):147-54. Mol Cancer Res. 2002. PMID: 12496361
-
Efficient serum-free derivation of oligodendrocyte precursors from neural stem cell-enriched cultures.Stem Cells. 2009 Jan;27(1):116-25. doi: 10.1634/stemcells.2007-0205. Stem Cells. 2009. PMID: 18403757 Free PMC article.
-
Growth factors and transcription factors in oligodendrocyte development.J Cell Sci Suppl. 1991;15:117-23. doi: 10.1242/jcs.1991.supplement_15.16. J Cell Sci Suppl. 1991. PMID: 1668594 Review.
-
Human neural stem cells: isolation, expansion and transplantation.Brain Pathol. 1999 Jul;9(3):499-513. doi: 10.1111/j.1750-3639.1999.tb00538.x. Brain Pathol. 1999. PMID: 10416990 Free PMC article. Review.
Cited by
-
Neural Plasticity in Multiple Sclerosis: The Functional and Molecular Background.Neural Plast. 2015;2015:307175. doi: 10.1155/2015/307175. Epub 2015 Jul 2. Neural Plast. 2015. PMID: 26229689 Free PMC article. Review.
-
Gene expression profiling of human neural progenitor cells following the serum-induced astrocyte differentiation.Cell Mol Neurobiol. 2009 May;29(3):423-38. doi: 10.1007/s10571-008-9338-2. Epub 2009 Jan 7. Cell Mol Neurobiol. 2009. PMID: 19130216 Free PMC article.
-
Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury.Mol Brain. 2013 Jan 8;6:3. doi: 10.1186/1756-6606-6-3. Mol Brain. 2013. PMID: 23298657 Free PMC article.
-
Rat Hippocampal Neural Stem Cell Modulation Using PDGF, VEGF, PDGF/VEGF, and BDNF.Stem Cells Int. 2019 Mar 18;2019:4978917. doi: 10.1155/2019/4978917. eCollection 2019. Stem Cells Int. 2019. PMID: 31011333 Free PMC article.
-
c-Cbl Regulates Murine Subventricular Zone-Derived Neural Progenitor Cells in Dependence of the Epidermal Growth Factor Receptor.Cells. 2023 Oct 3;12(19):2400. doi: 10.3390/cells12192400. Cells. 2023. PMID: 37830613 Free PMC article.
References
-
- Auffrey C, Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980;107:303–314. - PubMed
-
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank D, Rozovsky I, Stahl N, Yancopoulos G, Greenberg M. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. - PubMed
-
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neural survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
