Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy
- PMID: 11331382
- PMCID: PMC6762478
- DOI: 10.1523/JNEUROSCI.21-10-03531.2001
Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy
Abstract
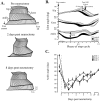
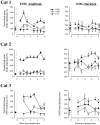
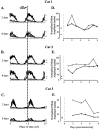
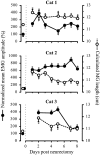
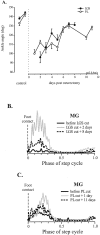
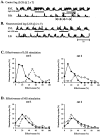
After lateral gastrocnemius-soleus (LGS) nerve section in intact cats, a rapid locomotor compensation involving synergistic muscles occurs and is accompanied by spinal reflex changes. Only some of these changes are maintained after acute spinalization, indicating the involvement of descending pathways in functional recovery. Here, we address whether the development of these adaptive changes is dependent on descending pathways. The left LGS nerve was cut in three chronic spinal cats. Combined kinematics and electromyographic (EMG) recordings were obtained before and for 8 d after the neurectomy. An increased yield at the ankle was present early after neurectomy and, as in nonspinal cats, was gradually reduced within 8 d. Compensation involved transient changes in step cycle structure and a longer term increase in postcontact medial gastrocnemius (MG) EMG activity. Precontact MG EMG only increased in one of three cats. In a terminal experiment, the influence of group I afferents from MG and LGS on stance duration was measured in two cats. LGS effectiveness at increasing stance duration was largely decreased in both cats. MG effectiveness was only slightly changed: increased in one cat and decreased in another. In cat 3, the plantaris nerve was cut after LGS recovery. The recovery time courses from both neurectomies were similar (p > 0.8), suggesting that this spinal compensation is likely a generalizable adaptive strategy. From a functional perspective, the spinal cord therefore must be considered capable of adaptive locomotor plasticity after motor nerve lesions. This finding is of prime importance to the understanding of functional plasticity after spinal injury.
Figures



Similar articles
-
Partial denervation of ankle extensors prior to spinalization in cats impacts the expression of locomotion and the phasic modulation of reflexes.Neuroscience. 2009 Feb 18;158(4):1675-90. doi: 10.1016/j.neuroscience.2008.11.005. Epub 2008 Nov 8. Neuroscience. 2009. PMID: 19056469
-
Plasticity in reflex pathways controlling stepping in the cat.J Neurophysiol. 1997 Sep;78(3):1643-50. doi: 10.1152/jn.1997.78.3.1643. J Neurophysiol. 1997. PMID: 9310449
-
Adaptive muscle plasticity of a remaining agonist following denervation of its close synergists in a model of complete spinal cord injury.J Neurophysiol. 2016 Sep 1;116(3):1366-74. doi: 10.1152/jn.00328.2016. Epub 2016 Jun 29. J Neurophysiol. 2016. PMID: 27358318 Free PMC article.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
The "beneficial" effects of locomotor training after various types of spinal lesions in cats and rats.Prog Brain Res. 2015;218:173-98. doi: 10.1016/bs.pbr.2014.12.009. Epub 2015 Mar 29. Prog Brain Res. 2015. PMID: 25890137 Review.
Cited by
-
Adaptation after vastus lateralis denervation in rats demonstrates neural regulation of joint stresses and strains.Elife. 2018 Sep 3;7:e38215. doi: 10.7554/eLife.38215. Elife. 2018. PMID: 30175959 Free PMC article.
-
Spinal cord injury: present and future therapeutic devices and prostheses.Neurotherapeutics. 2008 Jan;5(1):147-62. doi: 10.1016/j.nurt.2007.10.062. Neurotherapeutics. 2008. PMID: 18164494 Free PMC article. Review.
-
Differential modulation of crossed and uncrossed reflex pathways by clonidine in adult cats following complete spinal cord injury.J Physiol. 2012 Feb 15;590(4):973-89. doi: 10.1113/jphysiol.2011.222208. Epub 2012 Jan 4. J Physiol. 2012. PMID: 22219338 Free PMC article.
-
Functional recovery of aimed scratching movements after a graded proprioceptive manipulation.J Neurosci. 2009 Mar 25;29(12):3897-907. doi: 10.1523/JNEUROSCI.0089-09.2009. J Neurosci. 2009. PMID: 19321786 Free PMC article.
-
Locomotor changes in length and EMG activity of feline medial gastrocnemius muscle following paralysis of two synergists.Exp Brain Res. 2010 Jun;203(4):681-92. doi: 10.1007/s00221-010-2279-2. Epub 2010 May 11. Exp Brain Res. 2010. PMID: 20458472 Free PMC article.
References
-
- Armstrong RB, Marum P, Tullson P, Saubert CW. Acute hypertrophic response of skeletal muscle to removal of synergists. J Appl Physiol. 1979;46:835–842. - PubMed
-
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. - PubMed
-
- Bélanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol. 1996;76:471–491. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
